Резюме
Актуальність. Пошук факторів, що впливають на прогресування структурної перебудови слизової оболонки шлунка та канцерогенез, залишається перспективним напрямком гастроентерології. Мета дослідження: вивчити мікроструктуру слизової оболонки шлунка у хворих на хронічний атрофічний гастрит залежно від наявності структурних змін щитовидної залози. Матеріали та методи. Проведено дослідження гістоструктурних змін слизової оболонки шлунка в 121 хворого на атрофічний гастрит з урахуванням вузлових та дифузних змін у паренхімі щитовидної залози. Ступінь та стадію гастриту оцінювали за системою OLGA. Вивчали алергічний компонент, вираженість атрофічних змін у слизовій оболонці шлунка, наявність і вираженість склеротичних змін строми, гіперплазію епітелію. Ультразвукове дослідження щитовидної залози виконували на ультразвуковому сканері Toshiba Xario (Japan). Результати. Розподіл пацієнтів за частотою виявлення атрофії в різних локусах шлунка показав, що кишкову метаплазію в тілі шлунка у хворих із вузловими змінами спостерігали в 1,9 раза частіше, ніж у пацієнтів із дифузними змінами (χ2 = 5,33; р < 0,05), та в 2,8 раза — ніж в осіб без структурних змін щитовидної залози (χ2 = 8,12; р < 0,01). Аналогічно атрофію в куті шлунка найчастіше виявляли у хворих із вузловими змінами в щитовидній залозі — у 65,0 % випадків, що на 25,7 % частіше порівняно з пацієнтами з дифузними змінами (р > 0,05) та на 38,3 % — з особами без структурних змін щитовидної залози (χ2 = 6,27; р < 0,05). За результатами кореляційного аналізу виявлено зв’язок між наявністю вузлових змін у щитовидній залозі й ступенем атрофії слизової оболонки тіла шлунка (r = 0,49; р = 0,011) та кута шлунка (r = 0,52; р = 0,037), наявністю кишкової метаплазії в слизовій оболонці антрального відділу шлунка (r = 0,54; р = 0,013) та кута шлунка (r = 0,41; р = 0,028). Висновки. Прогресування структурних змін слизової оболонки шлунка найбільш часто відбувається в пацієнтів із вузловими змінами в щитовидній залозі. Для ранньої діагностики передракових станів та змін слизової оболонки шлунка хворим із структурними змінами щитовидної залози доцільно проводити ретельне ендоскопічне дослідження шлунка із застосуванням сучасних високоінформативних технологій.
Background. The search for factors that influence the progression of structural remodeling of the gastric mucosa and carcinogenesis remains a promising area of gastroenterology. The purpose of the study: to study the microstructure of the gastric mucosa in patients with chronic atrophic gastritis depending on the presence of structural changes in the thyroid gland. Materials and methods. A study of histostructural changes of the gastric mucosa was carried out in 121 patients with atrophic gastritis, taking into account nodular and diffuse changes in the parenchyma of the thyroid gland. The degree and stage of gastritis were assessed according to the OLGA system. We studied the allergic component, the severity of atrophic changes in the gastric mucosa, the presence and severity of sclerotic changes in the stroma, and epithelial hyperplasia. Ultrasound examination of the thyroid gland was performed on an ultrasound scanner Toshiba Xario (Japan). Results. The distribution of patients according to the frequency of detection of atrophy in different loci of the stomach showed that intestinal metaplasia in the body of the stomach was observed 1.9 times more often in patients with nodular changes than in those with diffuse changes (χ2 = 5.33; p < 0.05) and 2.8 times — than in people without structural changes of the thyroid gland (χ2 = 8.12; p < 0.01). Similarly, atrophy in the angle of the stomach was most often detected in patients with nodular changes in the thyroid gland — in 65.0 % of cases, which is 25.7 % more often compared to those with diffuse changes (p > 0.05) and 38.3 % more often compared to people without structural changes of the thyroid gland (χ2 = 6.27; p < 0.05). According to the results of the correlation analysis, a connection was found between the presence of nodular changes in the thyroid gland and the degree of atrophy of the mucous membrane of the body of the stomach (r = 0.49; p = 0.011) and the angle of the stomach (r = 0.52; p = 0.037), the presence of intestinal metaplasia in the mucous membrane of the antral part of the stomach (r = 0.54; p = 0.013) and the angle of the stomach (r = 0.41; p = 0.028). Conclusions. The progression of structural changes in the gastric mucosa occurs most often in patients with nodular changes in the thyroid gland. For early diagnosis of precancerous conditions and changes in the mucous membrane of the stomach in patients with structural changes of the thyroid gland, it is advisable to conduct a thorough endoscopic examination of the stomach using modern highly informative technologies.
Introduction
The urgency of the problem of chronic atrophic gastritis (CAG) is due to the proven fact that this pathology tends to progress with the gradual development of metaplastic and dysplastic changes in the epithelium of the gastric mucosa (GM) with the probable occurrence of intestinal gastric cancer. This multistage cascade of gastric carcinogenesis, known as the Correa cascade [1], has been repeatedly confirmed in various studies [2–4]. Although gastric cancer is declining in the developed world, it remains a serious problem today, largely due to the low level of early diagnosis [5, 6].
The use of modern endoscopic diagnostic methods, in particular, high-definition video endoscopy, with modes of magnification and narrowband imaging, allows with high probability to diagnose structural changes in the stomach and perform ultra-precise tissue sampling. However, to date, the main method of diagnosing the presence and degree of GM atrophy is histological examination. Gastric mucosa atrophy is characterized by deep diffuse inflammatory infiltration, dysregeneration of superficial epithelial cells, as well as a decrease in the number of normal glands (main and lining). Morphologically, atrophy is considered mild with loss of less than 30 % of glands, moderate — 30–60 %, severe — more than 60 % of glands [7, 8]. There is currently no generally accepted classification of atrophy severity from the standpoint of endoscopic examination.
Progression of atrophy to intestinal metaplasia (replacement of gastric epithelium by intestinal) is negative and requires careful monitoring. Intestinal metaplasia can be classified as complete (small intestine, or type I) or incomplete (small intestine, or type IIA/II, and colonic, or type IIB/III). The question of the feasibility of determining the subtypes of intestinal metaplasia is complex and controversial [9–11]. Some researchers argue that incomplete intestinal metaplasia (type III) is associated with an increased risk of gastric cancer, but these observations have not been confirmed in other studies [12]. Meanwhile, the work of Isajevs S. published in 2021 indicates a high prevalence of incomplete intestinal metaplasia, which was found not only in patients with advanced metaplasia, but also in those stratified by the Operative Link on Gastric Intestinal Metaplasia Assessment (OLGIM I) staging system. Without the identification of this subtype of intestinal metaplasia, patients at high risk of gastric cancer would be omitted for observation [4].
Since the genetic events that cause the development of intestinal metaplasia are still a mystery, and the mechanisms leading to the progression of structural rearrangement of gastric mucosa and carcinogenesis have not been definitively determined, we look for other factors and interactions that can initiate gastric pathology. In this sense, we found it quite interesting to study the condition of the thyroid gland in patients with chronic atrophic gastritis, as an organ that has some embryological, biochemical and genetic commonality with the stomach. It is this commonality that can determine the only pathogenetic mechanisms for the formation of some thyroid and gastric conditions, in particular, autoimmune diseases [13]. Isolated experimental and clinical studies indicate an association between nodular thyroid changes, iodine deficiency, and the development of gastric adenocarcinoma [14].
Thus, there are very few studies investigating the relationship between CAG and pathological changes in the thyroid gland, they are contradictory and outdated.
The purpose of our study is to investigate the microstructure of the gastric mucosa in patients with chronic atrophic gastritis depending on the presence of structural changes in the thyroid gland.
Material and methods
The material for the study of histostructural changes in gastric mucosa were samples obtained during gastroscopy (from the body of the stomach and antrum of the stomach 2 biopsies of lesser and greater curvature, and 1 biopsy from the gastric angle). Biopsies were fixed in 10.0% solution of neutral buffered formalin, dehydrated in alcohols of ascending concentration and poured into paraffin. Histological sections 5 μm thick were stained with hematoxylin and eosin. The degree and stage of gastritis were assessed in the obtained histological specimens according to the OLGA (Operative Link for Gastritis Assessment, 2008) system. The activity of gastritis was determined by the degree of infiltration of lamina propria by neutrophils, and the severity of chronic inflammation — by the degree of lymphoplasmacytic infiltration. The presence of an allergic component (eosinophilic leukocytes), atrophic changes in the central nervous system (reduction of the number of specific parenchymal cells due to a decrease in their absolute number or metaplastic transformation and pseudopylorization of the fundus glands) were also described, as well as the presence and severity of sclerotic changes in the stroma, epithelial hyperplasia.
Ultrasound examination of the thyroid gland was performed on an ultrasound scanner Toshiba Xario (Japan) using a multi-frequency linear transducer with a frequency of 5–12 MHz.
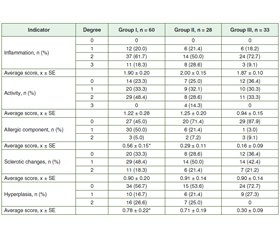
Selection criteria for examination were structural changes in the gastric mucosa — atrophy and/or intestinal metaplasia. The study included 121 patients with chronic atrophic gastritis, which were divided into 3 groups depending on the changes in thyroid structure: group I consisted of 60 people with nodular changes in the thyroid gland, II — 28 patients with diffuse changes in the thyroid gland, III — 33 people without changes in thyroid structure.
Statistical data processing was performed using SPSS 19.0. To describe the quantitative features, we used mean arithmetic (х) and standard errors (SE). To assess the differences between the quantitative parameters in the groups, we used the Tukey test with Bonferroni correction. Fi–sher’s criterion (F) and χ2 were used for qualitative data. To analyze the interrelation between the parameters, we used correlation analysis with consideration of Spearman rank coefficients (ρ). The indicator of the level of statistical significance for all species in the analysis accounted for 5 %. The differences were considered statistically significant if the probability of occurrence of differences did not exceed 0.05 (p < 0.05).
Results and discussion
The histological study of patients with CAG has shown that stage I and stage II gastritis according to the OLGA system were the most common, but the range of values was quite wide (Table 1).
In the group of patients without structural changes in the thyroid gland, the depth of lymphohistocytic infiltration of the gastric mucosa stroma extended beyond the surface epithelium and reached the secretory epithelium, sometimes reaching the GM muscle plate. The degree of inflammatory changes was slightly higher in the antrum of the stomach (p < 0.05) (Tables 2, 3).
/30.jpg)
Inflammatory activity in patients without structural changes in the thyroid gland was weak and moderate with slightly higher rates in the antrum. In one patient, eosinophilic granulocytes diffusely located in the stroma in small numbers among the cells of the inflammatory infiltrate in biopsies from the body of the stomach and in two patients — in the antrum. Lymphoid follicles located among the spread glands were found in 12.1 % of patients. Lesions of the glands led to a decrease in the height of the secretory department, and, as in the case of inflammation, atrophic changes dominated in the antrum and exceeded this figure in biopsies from the body of the stomach twice. Weak focal or diffuse fibrosis of the stroma is detected both in the body of the stomach and in the antrum.
Morphological changes in the surface foveolar epithelium in patients with diffuse thyroid changes were determined in the form of dystrophic changes in the cylindrical epithelium and vacuolation of the cytoplasm. Inflammatory lymphohistiocytic infiltration was diffuse in nature, extending deep into the mucosa and reaching the muscle plate, pushing apart the glands. Characterizing the specifics of localization and severity of inflammation in patients with CAG and diffuse thyroid changes, it should be noted that the average density of inflammatory infiltrate in the antrum was higher by 8.5 %, where high activity of inflammation was twice as common. The content of eosinophilic granulocytes among inflammatory infiltrate cells in the gastric mucosa exceeded by 33.3 % that of the antrum of the stomach. The process of gland loss in the secretory department was more often characterized by moderate atrophic changes, in the antrum it was 5.3 % higher than in the mucosa of the gastric body. In half of the patients, there was a total atrophy of the mucous membrane of the antrum of the stomach on the background of pseudopylorization of the pyloric glands (Fig. 1). Hyperplasia was found in the body of the stomach in 32.2 %, in the antrum — in 46.4 % of patients, the average in the latter was higher by 54.9 %.
/30_2.jpg)
In patients with СAG and nodular changes of the thyroid gland, the integumentary foveolar epithelium consisted of smoothed rollers and tortuous fossae, some of which are branched. The epithelium was variomorphic — from high prismatic to low cubic, with signs of impaired maturation. Inflammatory changes in patients with СAG and nodular changes in the thyroid gland differed in intensity depending on the location. Thus, in biopsies from the body of the stomach, the inflammation in 90.0 % of patients was characterized as moderate or severe, while in the antrum it was moderate and mild, the difference between the severity of inflammation was 15.4 %. Indicators of activity also prevailed in the body of the stomach, starting from moderate severity. A small number of eosinophilic granulocytes in the stomach was detected in 35.0 % of patients and in the antrum — in 55.0 %. Atrophy was manifested not only by a decrease in the absolute number of glandular cellular elements, but also by the replacement and displacement of specialized cells by intestinal epithelium in metaplasia. Stromal fibrosis was almost equally pronounced in both parts of the stomach. Hyperplasia of the integumentary epithelium and pseudopylorization of the fundic glands of the stomach were detected in patients with nodular thyroid changes: in 46.7 % of cases — in the body of the stomach and in 43.3 % of cases — in its antrum (Fig. 2).
Only among patients with nodular changes in the thyroid gland, there were isolated cases when on the background of inflammatory changes, the integumentary epithelium had an immature appearance with weak signs of regenerative dysplasia in the form of pseudomultilayered changes in cell shape to low prismatic or cubic, nuclear hyperchromia (Fig. 3).
The distribution of patients by the frequency of atrophy in different loci of the stomach showed that those with nodular thyroid changes were 1.5 times more likely to have atrophy in the body of the stomach than people with diffuse thyroid changes (p > 0.05) and 2.4 times more often — than patients without structural changes of the thyroid gland (p < 0.05) (Fig. 4).
Similarly, intestinal metaplasia in patients with nodular thyroid changes was 1.9 times more common than in those with diffuse thyroid changes (χ2 = 5.33; p < 0.05), and 2.8 times — than in patients without structural changes of the thyroid gland (χ2 = 8.12; p < 0.01).
The findings of such an area as the angle of the stomach, which in recent years has received considerable attention as a marker locus in the diagnosis of precancerous changes and in assessing the risk of their detection, are important. Thus, according to our data, in general, morphological changes in the form of atrophy were most often found in patients with nodular changes in the thyroid gland — in 65.0 % of cases, which is 25.7 % more often than in those with diffuse changes in the thyroid gland (p > 0.05) and 38.3 % more often compared to people without structural changes of the thyroid gland (χ2 = 6.27; p < 0.05). Similarly, intestinal metaplasia in the angle of the stomach in patients with nodular changes was observed 1.4 times more often than in those with diffuse changes in the thyroid gland (p > 0.05) and 5.7 times more often than in people without thyroid pathology (F = 0.043; p < 0.05).
Correlation analysis revealed a relationship between the presence of nodular changes in the thyroid gland and the degree of GM atrophy in the gastric body (r = 0.49; p = 0.011) and the angle of the stomach (r = 0.52; p = 0.037), the presence of intestinal metaplasia in the antrum of the stomach (r = 0.54; p = 0.013) and the angle of the stomach (r = 0.41; p = 0.028).
To date, there is no doubt about the relevance of early diagnosis of precancerous conditions and changes in the gastric mucosa. Our study showed that the progression of structural changes in the stomach occurs most often in patients with nodular changes in the thyroid gland, as evidenced by the identified correlations. The obtained data coincide with the previously presented hypothesis about the embryological commonality of the stomach and thyroid gland. Given the results of the study in patients with structural changes in the thyroid gland, it is advisable to conduct a thorough endoscopic examination of the stomach using modern highly informative technologies.
Conclusions
1. The frequency and severity of morphological changes in the gastric mucosa was significantly higher in patients with СAG and nodular changes in the thyroid gland compared to those with diffuse changes or people without thyroid pathology.
2. Patients with CAG are recommended to conduct a sonological examination of the thyroid gland to exclude pathological changes in it. On the other hand, when detecting diffuse and/or nodular changes of the thyroid parenchyma, it is advisable to conduct a thorough endoscopic examination of the stomach, if necessary, with histological examination of biopsies.
Prospects for further research are to determine the diagnostic informativeness of indicators of sonological examination of the thyroid gland to predict structural changes in the gastric mucosa in patients with atrophic gastritis.
Received 22.07.2022
Revised 05.08.2022
Accepted 16.08.2022
/30_2.jpg)

/29.jpg)
/30.jpg)
/31.jpg)
/31_2.jpg)