Журнал "Гастроэнтерология" Том 55, №4, 2021
Вернуться к номеру
Вивчення тканинного IgG4 у слизовій оболонці товстої кишки в пацієнтів iз запальними захворюваннями кишечника
Авторы: Yu.M. Stepanov, T.S. Tarasova, M.V. Stoikevych, Yu.A. Gaydar, N.S. Fedorova
State Institution “Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine”,
Dnipro, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
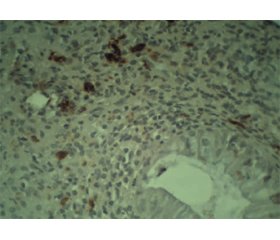
Актуальність. На сьогодні запальні захворювання кишечника (ЗЗК) є глобальною проблемою, поширеність якої зростає в усьому світі. Це значно підвищує економічне навантаження на систему охорони здоров’я. Останнім часом у багатьох дослідженнях вказують на важливу роль імуноглобуліну G4 (IgG4) у формуванні хронічного запалення при ЗЗК і можливість використання його як біомаркера запального процесу. Мета: удосконалити діагностику хронічних запальних захворювань кишечника на підставі вивчення стану IgG4-позитивних плазматичних клітин у слизовій оболонці товстої кишки в пацієнтів із виразковим колітом (ВК) та хворобою Крона (ХК). Матеріали та методи. Обстежено 34 осіб із ЗЗК: 25 із ВК та 9 із ХК, із них 20 жінок та 14 чоловіків середнім віком (38,8 ± 3,0) та (38,2 ± 3,7) року відповідно. Пацієнти розподілені на групи залежно від нозології та тяжкості перебігу захворювання. В усіх хворих виконане ендоскопічне дослідження товстої кишки з метою встановлення або уточнення діагнозу, отримані біопсійні зразки для гістологічного та імуногістохімічного аналізу. Результати. У 13 (38,3 %) з 34 обстежених пацієнтів виявлений позитивний результат на наявність тканинного IgG4 (≥ 10 клітин у полі зору). Серед осіб із ВК 48,0 % мають позитивний результат імуногістохімічного дослідження на тканинний IgG4, у пацієнтів iз ХК цей показник становить 11,1 %, що дає нам можливість стверджувати, що при ВК підвищення тканинного IgG4 зустрічається в 4,4 раза частіше. При середньому ступені тяжкості ВК позитивний тканинний IgG4 виявляють в 1,1 раза частіше, ніж при тяжкому перебігу. У хворих із легким ступенем тяжкості тканинний IgG4 не виявлявся. Висновки. При ВК IgG4-позитивні клітини в слизовій оболонці товстої кишки зустрічаються частіше, ніж при ХК, що дає можливість використовувати цей показник для диференційної діагностики виразкового коліту і хвороби Крона. Позитивний тканинний IgG4 зустрічається частіше при середньому ступені тяжкості, ніж при тяжкому.
Background. Inflammatory bowel disease (IBD) is a global problem today, with a growing prevalence in the world. It significantly increases the economic burden on the health care system. Recently, many studies indicate the important role of immunoglobulin G4 (IgG4) in the formation of chronic inflammation in IBD and the possibility of using it as a biomarker of the inflammatory process. The purpose was to improve the diagnosis of chronic inflammatory bowel diseases by studying the status of IgG4-positive plasma cells in the mucous membrane of the colon in patients with ulcerative colitis (UC) and Crohn’s disease (CD). Materials and methods. We have examined 34 patients with IBD, 25 with UC and 9 with CD, of them 20 women and 14 men, with an average age of (38.8 ± 3.0) and (38.2 ± 3.7) years, respectively. Patients were divided into groups depending on the nosology and severity of the disease. All patients underwent endoscopic examination of the colon to establish or clarify the diagnosis, and biopsy specimens were taken for histological and immunohistochemical examination. Results. In 13 (38.3 %) of 34 examined patients, a positive result for the presence of tissue IgG4 (≥ 10 cells in the field of view) was found. Among patients with UC, 48 % have a positive result of immunohistochemical examination of tissue IgG4, in people with CD, this figure is 11.1 %. This gives us reason to say that in UC, elevation of tissue IgG4 levels occurs 4.4 times more often. Positive tissue IgG4 in patients with moderate UC was found 1.1 times more often than in severe UC. Among patients with mildly active disease, tissue IgG4 was not detected. Conclusions. In UC, IgG4-positive cells in the mucous layer of the colon are more common than in CD, which makes it possible to use this indicator for the differential diagnosis of ulcerative colitis and Crohn’s disease. Positive tissue IgG4 is more common in moderate form than in severe one.
запальні захворювання кишечника; виразковий коліт; хвороба Крона; IgG4
inflammatory bowel diseases; ulcerative colitis; Crohn’s disease; IgG4
Introduction
Materials and methods
/40.jpg)
Results and discussions
/42.jpg)
Conclusions
- Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nature Reviews Gastroenterology & Hepatology. 2015. 12(12). 720-727. doi: 10.1038/nrgastro.2015.150.
- Sub Lee H., Jae Lee K. Immunoglobulin G4-related immune responses to common food antigens in patients with ulcerative colitis and Crohn’s disease. Turkish Journal of Gastroenterology. 2019. 5(5). 408-414. doi: 10.5152/tjg.2019.18466.
- Ng S.C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018. 390. 2769-2778. doi: 10.1016/S0140-6736(17)32448-0.
- Lee S.H., Kwon J., Cho M.L. Immunological pathogenesis of inflammatory bowel disease. Intestinal Research. 2018. 16(1). 26. doi: 10.5217/ir.2018.16.1.26.
- Šimurina M. et al. Glycosylation of immunoglobulin G associa–tes with clinical features of inflammatory bowel diseases. Gastroenterology. 2018. 154(5). 1320-1333. doi: 10.1053/j.gastro.2018.01.002.
- British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019. 68(3). 1-106. doi: 10.1136/gutjnl-2019-318484.
- Dietary guidance for patients with inflammatory bowel disease from the International Organization for the Study of Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology. 2020. 18. 1381-1392. doi: 10.1016/j.cgh.2020.01.046.
- Limketkai B.N., Wolf A., Parian A.M. Nutritional interventions in the patient with inflammatory bowel disease. Gastroenterology Clinics of North America. 2018. 47(1). 155-177. doi: 10.1016/j.gtc.2017.09.007.
- Guan Q.A. Comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019 Dec 1. 2019. 7247238. doi: 10.1155/2019/7247238.
- Armstrong H. et al. Host immunoglobulin G selectively identifies pathobionts in pediatric inflammatory bowel disease. Microbiome. 2019. 7(1). 1-17. doi: 10.1186/s40168-018-0604-3.
- Wang Z. et al. High level of IgG4 as a biomarker for a new subset of inflammatory bowel disease. Scientific Reports. 2018. 8(1). 10018. doi: 10.1038/s41598-018-28397-8.
- European consensus on the histopathology of inflammatory bowel disease. Journal of Crohn’s and Colitis. 2013. 7(10). 827-851. doi: 10.1016/j.crohns.2013.06.001.
- Smids C. et al. The value of serum antibodies in differentiating inflammatory bowel disease, predicting disease activity and disease course in the newly diagnosed patient. Scandinavian Journal of Gastroenterology. 2017. 52(10). 1104-1112. doi: 10.1080/00365521.2017.1344875.
- Dmochowska N., Wardill H.R., Hughes P.A. Advances in imaging specific mediators of inflammatory bowel disease. International Journal of Molecular Sciences. 2018. 19(9). 2471. doi: 10.3390/ijms19092471.
- Šimurina M. et al. Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology. 2018. 154(5). 1320-1333. doi: 10.1053/j.gastro.2018.01.002.
- Chen X. et al. IgG4+ plasma cell infiltration is correlated with the development of inflammatory bowel disease and can be regulated by TLR-4. Int. J. Clin. Exp. Pathol. 2018. 11(9). 4537-4544.
- Trampert D.C. et al. On the role of IgG4 in inflammatory conditions: lessons for IgG4-related disease. Biochimica et Biophysica Acta — Molecular Basis of Disease. 2018. 1864(4). 1401-1409. doi: 10.1016/j.bbadis.2017.07.038.
- Harkness T. et al. Immunoglobulin G and immunoglobulin G subclass concentrations differ according to sex and race. Annals of Allergy Asthma Immunology. 2020. 125. 190-195. doi: 10.1016/j.anai.2020.03.018.
- Lewis J.D., Chuai S., Nessel L., Lichtenstein G.R., Aberra F.N. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflam. Bowel Dis. 2008. 14(12). 1660-1666. doi: 10.1002 ibd.20520.
- Lobatón T., Bessissow T., De Hertogh G., Lemmens B., Maedler C. The Modified Mayo Endoscopic Score (MMES): a new index for the assessment of extension and severity of endoscopic activity in ulcerative colitis patients. J. Crohn’s Colitis. 2015 Oct. 9(10). 846-52. doi: 10.1093/ecco-jcc/jjv111.
- Restellini S., Chao C.-Y., Martel M., Affif W. Clinical parameters correlate with endoscopic activity of ulcerative colitis: a systematic review. Clinical Gastroenterology and Hepatology. 2019. 17(7). 1265-1275.e8. doi: 10.1016/j.cgh.2018.12.021.
- Gionchetti P., Dignass A., Danese S., Magro F.J. Dias. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016. Part 2: surgical management and special situations. Journal of Crohn’s and Colitis. 2017. 11(2). 135-149. doi: 10.1093/ecco-jcc/jjw169.
- Vashist M.N., Samaan M., Mosli M.H., Parker C.E. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst. Rev. 2018 Jan 16. 1(1). CD011450. doi: 10.1002/14651858.CD011450.pub2.
- Петри А., Сэбин К. Наглядная статистика в медицине. Москва: Гэотар-Мед, 2003. 143 с.
- Иванова В.С. Основы математической статистики. Москва, 1990. 174 с.
- Енюков И.С. Методы, алгоритмы, программы многомерного статистического анализа. Москва: Финансы и статистика, 1986. 86 с.

/41.jpg)
/41_2.jpg)