Introduction
Bacterial infections are being discussed as the most important complications of end-stage liver disease in terms of frequency, severity, cost of services and adverse outcomes due to developing acute decompensation of cirrhosis associated with infectious process [1, 2]. In addition, bacterial infection is a common acute decompensating event in liver cirrhosis, which may result in acute-on-chronic liver failure (ACLF), the syndrome characterized by hepatic and extrahepatic organ failure and associated with high short-term mortality [3, 4].
European Association for the Study of the Liver (EASL) — Chronic Liver Failure (CLIF) Consortium and the European Foundation for the Study of Chronic Liver Failure have defined the ACLF criteria, and also designed grades of severity based on the Sequential Organ Failure Assessment (CLIF-SOFA) score [3, 5]. It has been shown that ACLF is most frequently associated with bacterial infections [6, 7]. Episodes of spontaneous bacterial peritonitis or pneumonia were more often associated with the development of ACLF compared to infections of other localizations [3, 7].
It should be noted that the definition of ACLF syndrome has not yet been unified among continental associations for the study of liver diseases [3, 8, 9].
Currently, ACLF is the subject of numerous studies [3, 4, 7, 9]. Leading experts in this field (R. Moreau, P.S. Kamath, R. Jalan, P. Ginès, G. García-Tsao, B. Schnabl, S. Piano) consider ACLF the most severe form of acute decompensation in cirrhosis [1], and the field of research related to ACLF is prospective. In 2000, one thematic article was published, when in 7 months of 2021, 475 papers were already published. The features of ACLF forms are being studied in subgroups of patients with single kidney failure, failure of any organ other than kidney (non-kidney ACLF), of a single organ with serum creatinine level > 133 μmol/L, with primary liver injury (for example, due to alcohol or acute viral hepatitis B) and influence of common complications (eg, spontaneous bacterial peritonitis, sepsis) [10, 11].
We attempted to assess cirrhosis decompensation in gastroenterology unit patients with bacterial infections and complications according to the ACLF criteria. For this purpose, a retrospective analysis was performed of possible risk factors and consequences of ACLF syndrome in a group of patients admitted to the gastroenterology unit of a city hospital.
The purpose of the study was to assess the decompensation of cirrhosis in patients with bacterial infections based on the CLIF-C score.
Materials and methods
Retrospective data analysis was performed of all cirrhotic patients consecutively admitted to the gastroenterology unit of a city clinic between 2011 and 2014. Three patients were excluded from the study: in two patients, along with cirrhosis, a malignant neoplasm with peritoneal carcinomatosis was diagnosed, and in one person we missed the data necessary for the ACLF score.
The study included 151 cirrhotic patients, 84 males and 67 females. Their age varied from 25 to 76 years, with median (Me) of 55 (Q1 = 43; Q3 = 61) years. Laboratory and instrumental studies were performed in accordance with the clinical protocol for the diagnosis and treatment of patients with digestive system diseases. Multidrug-resistant bacteria were defined as strains resistant to at least three of the main antibiotic classes, including beta-lactam antibiotics.
The syndrome of acute-on-chronic liver failure was diagnosed based on the criteria proposed by R. Moreau et al. using the Chronic Liver Failure-Consortium (CLIF-C) score, created on the basis of the original SOFA (Vincent J.L. et al., 1996) score [6]. The Child-Pugh and ACLF scores were used at the time of admission for all patients and in case of deterioration of the patient’s condition. Severity of hepatic encephalopathy (HE) was assessed using the West Haven criteria [12].
Precipitating factors and decompensating events were determined according to the CANONIC study [6]. The accepted period to assess the influence of the precipitating event on hospitalization with acute decompensation was 3 months. Alcohol as a trigger was defined as a significant consumption of alcohol within the last 3 months before admission.
Statistical processing of the research results was performed in the Windows XP operating system using the Statistica 6.0 software package (StatSoft, GS-35F-5899H; USA) and MedCalc (version 9.6.2.0; Belgium). The distribution of quantitative traits was assessed using the Shapiro-Wilk test and Levene’s test for equality of variances. Since the data differed from the normally distribution, nonparametric statistical methods were used. The median, minimum (Min) and maximum (Max) values, 25 (Q1) and 75 (Q3) percentiles, 95% confidence interval (95% CI) were calculated. When analyzing the primary data, pairwise comparison of independent samples was performed according to quantitative or ordinal attribute using the Mann-Whitney U test. When analyzing the qualitative (binary) attribute of two independent samples, we used a two-tailed test of Fisher’s exact test, χ2 and χ2 with Yates’ correction for continuity. For assessing risk factors, number needed to harm (NNH) was calculated. To determine the informative value of research methods, receiver operating characteristic (ROC) curves were plotted. The results were considered statistically significant at p < 0.05.
Results
Among 151 patients, 44 met ACLF criteria (29.1 %; 95% CI 22.0–37.1) while assessing cirrhosis manifestations. Median age was 55 years, 56.8 % of patients were males. The basic parameters of patients with ACLF are presented in Table 1.
Median Child-Pugh score at admission was 11. Among patients with ACLF, 98 % had hepatic encephalopathy. Ascites as a decompensating event was detected in 96 % of patients, all of them had grade 1 to 3 (as the first episode of ascites or a recurrent episode).
More than half of patients had oesophago-gastric varices (59 %). In 23 %, upper gastrointestinal bleeding was the cause of urgent hospitalization.
Potential precipitating events were as follows: bacterial infections — 25 patients (57 %), alcohol — 17 (39 %), upper gastrointestinal (GI) bleeding — 10 (23 %). In some cases, combinations of possible triggers have been identified. Despite the differences, we tried to compare some of our findings with those in the CANONIC study. In the CANONIC study, no precipitating event was identified in 43.6 % of patients with ACLF. At the same time, there were no patients in our cohort for whom potential triggers were not found and 41.0 % had more than one precipitating event (vs 13.5 % in the CANONIC study). It is difficult to explain; the clue probably lies in the approach to evaluating the triggers. According to our data, the main triggers were bacterial infections (57.0 versus 32.6 %) but can any episode of bacterial infection be a trigger? Among our patients with ACLF, the most common bacterial complication was urinary tract infection (UTI) — 30 % (half of the cases was isolated urinary infection).
The prevalence of ACLF grades in our patients and the CANONIC study was similar — grade 1 (20 and 11 %) predominated over grade 3 (4.6 and 3.5 %). Most often our patients had liver failure (70 %) that was defined by an increase in serum bilirubin level of 206 μmol/L (> 12 mg/dl), according to the ACLF criteria. Kidney failure was diagnosed in 12 patients (27 %). A rather high frequency of ACLF with kidney failure should be noted — the so-called kidney-ACLF, which is considered an unfavorable form of ACLF, even with its low total score [11].
Circulatory failure and HE were less common — each of the syndromes was observed in one of four patients with ACLF or 7.3 % in the total group. Coagulation disorders and respiratory failure were present in minority of patients.
Bacterial infections are considered major complications of cirrhosis due to immune abnormalities related to advanced liver disease [11]. The frequency of detection of infections is associated with the sensitivity of diagnostic methods, as well as with clinician’s awareness of these complications. We investigated the maximum possible loci of bacterial infection when the condition worsens, regardless of inflammation signs (fever, leukocytosis, etc.). This probably explains the frequency of diagnosed bacterial infections in people with ACLF (57 %) and all included patients (44.4 %; 95% CI 36.5–52.3).
The most common infection was UTI — 30 % (20.5 % of all patients). A microbiological agent was identified in 76 % of UTI cases. The predominant uropathogens were Gram-negative microorganisms (12/17; 70 %) of which in half of the cases, Escherichia coli was detected. Gram-positive bacteria in 4 of 5 cases were Enterococcus faecalis. Among uropathogens, multidrug-resistant bacteria were detected (24 %) such as Acinetobacter spp., Escherichia coli, Pantoea agglomerans, Enterococcus faecalis. These strains were resistant to the third-generation cephalosporins, which are most commonly used for empirical therapy of community-acquired infections in our unit. UTI pathogens were sensitive to fluoroquinolones in 94 % of cases. One of the important observations is that we didn’t find a significant number of cases of quinolone-resistant Enterobacteriaceae.
Microbiological agents were identified in 7 of 21 (24.0 %) blood samples and in 3 of 57 (5.0 %) ascitic fluid samples. Microorganisms isolated from ascitic fluid were Raoultella terrigena, Streptococcus pyogenes, Staphylococcus xylosus.
The most common microorganisms isolated in bloodstream infections were Gram-positive bacteria (Staphylococcus species), in two cases — methicillin-resistant staphylococci. Gram-negative bacteria were detected in one case (Escherichia coli). Median length of hospital stay was 23 (15–28) days.
Further, we compared baseline characteristics in patients with and without ACLF (Table 2).
In compared groups, there were no statistically significant differences in sex, age of patients, and etiology of cirrhosis. Not surprisingly, in the ACLF group, the median Child-Pugh score was statistically significantly higher (11 versus 9) than in patients without ACLF (p < 0.001). HE grade 1–2 and varicose veins were equally common in compared groups, while HE grade 3–4 and the incidence of ascites were higher in patients with ACLF (p = 0.026 and p = 0.006, respectively).
There were more cases of bacterial infections among patients with ACLF (p = 0.048). At the same time, no differences were found when analyzing the types of infections in ACLF and non-ACLF groups. In 13 patients (8.6 %; 95% CI 4.7–14.3), infections were classified as nosocomial. The median incubation period for hospital-acquired infections was 13 days (Min = 3, Max = 25; Q1 = 10; Q3 = 17). There was no difference in the frequency of these episodes.
Bleedings from the upper GI tract were found to be more frequent in patients with ACLF (22.7 versus 7.0 %, p = 0.010), including variceal haemorrhage (13.6 versus 1.9 %, χ2 = 7.85, p = 0.008). Calculations showed that the risk of developing ACLF in people with bleeding from the upper GI tract is increased with a likelihood of 4.2 (95% CI 1.5–11.9, χ2 = 6.64, p = 0.010) and for variceal haemorrhage — with a likelihood of 8.3 (95% CI 1.6–42.8, p = 0.008). On average, one of three individuals with cirrhosis and bleeding from the upper GI tract develops ACLF (NNH = 3.3; 95% CI 2.2–4.4).
Patients with ACLF had lower mean arterial pressure and were more likely to be dependent on vasopressors while in the intensive care unit. They also had a higher white cell count, higher total bilirubin and serum creatinine levels than those without ACLF.
For a more detailed assessment of the role of bacterial infections in the development of ACLF, a comparative analysis was performed of ACLF grades in 67 patients with infections and 84 individuals without them (Table 3).
The analysis showed that ACLF developed more frequent (37.7 versus 22.6 %) in patients with infections (χ2 = 3.90, p = 0.048). NNH was 6.8 (95% CI 6.1–7.5). Thus, on average, one in seven people with cirrhosis and infectious complication develops ACLF. Bacterial infections are considered the most significant risk factors of ACLF [12]. Analyzing ACLF grades in groups, it was found out that the proportion of individuals with ACLF-1 did not differ in the groups but more severe forms (grades 2 and 3) were observed more frequently in patients with infections. The risk of developing ACLF-2 and ACLF-3 in people with infections was 8.2 (95% CI 1.0–69.6; NNH = 12.9; 95% CI 10.7–15.0). According to the literature, it is ACLF-2 and ACLF-3, in contrast to ACLF-1, that are considered the most unfavorable forms of cirrhosis decompensation [13, 14].
We sought to compare the accuracy of the CLIF-C score with the most traditional model used in clinical practice (Child-Pugh score) to predict in-hospital mortality.
At the first stage, the comparative analysis of the scores for predicting in-hospital mortality was performed in the general group of patients with cirrhosis. Death was considered a primary endpoint. To determine the informative value of the scores under study, ROC curves were plotted. The diagnostic significance of this method was determined by the height of the ROC curve with the determination of the area under the curve (AUC). The point closest to the inflection point of the graph was taken as the cut-off one. Variables were assessed upon admission to the hospital and when the patient’s condition deteriorated (Fig. 1).
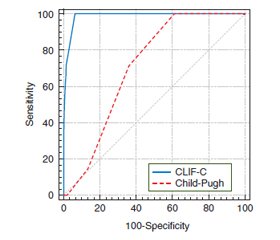
When predicting in-hospital mortality in the general group according to the Child-Pugh score, the following results were obtained: sensitivity — 100 % (95% CI 58.9–100), specificity — 38.9 % (95% CI 30.9–47.4). When using the CLIF-C score, sensitivity was 100 % (95% CI 58.9–100), specificity — 93.75 % (95% CI 88.5–97.1). At the second stage, diagnostic significance of the Child-Pugh and CLIF-C scores was compared to predict in-hospital mortality in patients with cirrhosis and bacterial infections (Fig. 2).
When predicting in-hospital mortality in a group of patients with cirrhosis and bacterial infections using the Child-Pugh score, the results were as follows: sensitivity — 100 % (95% CI 54.1–100), specificity — 29.5 % (95% CI 18.5–42.6). When using the CLIF-C score, sensitivity was 100 % (95% CI 58.9–100), specificity — 88.5 % (95% CI 77.8–95.2). Comparison of the AUC of the Child-Pugh and CLIF-C scores in patients with cirrhosis and infections are presented in Table 4.
/35_2.jpg)
According to the CLIF-C score, AUC corresponded to a model of very good quality in the group with cirrhosis and patients with cirrhosis and infections (0.99 and 0.97, respectively). This figure is statistically significantly higher compared to the traditionally used Child-Pugh score, which was demonstrated both in the general group of cirrhotic patients and in the group of patients with cirrhosis and infections (p = 0.012 and p = 0.015, respectively). Thus, the CLIF-C score has advantages over the Child-Pugh score when predicting in-hospital mortality for cirrhosis patients, including those with infectious complications.
Discussion
Currently, several prognostic models are used in clinical practice — Child-Pugh score, Model for End-Stage Liver Disease; to predict specific clinical situations — Maddrey’s discriminant function, Lille model, Glasgow score (for predicting the course of alcoholic hepatitis), prognostic index of Wilson’s disease manifested in acute liver failure [15–17]. In fact, any of these prognostic scales has certain limitations and is not considered ideal for predicting mortality.
In our country, in everyday practice, the most commonly used predictive model is the Child-Pugh score. The Child-Turcotte classification and its subsequent modification (Child-Turcotte-Pugh) represent an early empirical method for assessing the functional reserve of the liver among candidates for portosystemic shunting [17, 18]. Formally, the Child-Turcotte-Pugh score did not have a statistical assessment of accuracy but was extremely useful for stratifying patients with cirrhosis into risk groups. The score was used to evaluate the effectiveness of interventions such as transjugular intrahepatic portosystemic shunting and sclerotherapy [19, 20]. Despite the adequacy of the Child-Turcotte-Pugh score in assessing the degree of cirrhosis decompensation and patients’ survival, two of its parameters are subjective to some extent (ascites and HE) and limit the discriminatory ability [19]. An objective parameter such as the serum albumin concentration can be changed after albumin infusions. The value of prothrombin time varies if it is detected in different laboratories, which limits the possibility of comparing indicators [20]. In addition, the Child-Pugh score does not include prognostic factors not related to liver function (cardiac, renal, respiratory function, acid-base status). For example, the level of serum creatinine has crucial significance in predicting the outcome in cirrhosis [18]. Therefore, according to S. Fasolato et al. (2007), the Child-Pugh score has no predictive value for renal failure induced by bacterial infections [21].
Leading researchers in the field (V. Arroyo et al.; EASL-CLIF Consortium CANONIC Study) consider acute-on-chronic liver failure a new syndrome that can lead to cirrhosis reclassification [22]. The main result of the CANONIC study was the creation of the CLIF-C score for stratifying cirrhotic patients; the number of decompensated organs/systems (liver, kidneys, brain, coagulation, blood circulation, lungs) is taken into account when using CLIF-C score; it is valid for predicting outcomes in European patients with cirrhosis. In May 2015, in Bucharest, Romania, the EASL held a monothematic conference “Liver disease in resource-limited settings”, which attracted a large number of doctors from Eastern Europe and some regions of Africa. Since then, the term “resource-limited settings” has been increasingly found in scientific publications. But, in our opinion, these are not always limited resources but rather special conditions for which it is necessary to determine the applicability of diagnostic criteria, therapeutic methods, etc.
The results of our study demonstrated that the diagnostic significance of the CLIF-C score in terms of predicting in-hospital mortality in cirrhosis was higher compared to the Child-Pugh score. The CLIF-C score can be recommended for stratification of patients with advanced cirrhosis since it is an important step that provides an opportunity to redefine intensive care. This is due to the fact that the CLIF-C score takes into account more parameters that determine the prognosis for a selected group of patients, including parameters for assessing kidney dysfunction, respiratory and circulatory failure, and a more perfect standardized index of the coagulation system.
Conclusions
The frequency of ACLF syndrome in patients with cirrhosis in our gastroenterology unit is 29.1 %. The frequency of ACLF among individuals with bacterial infections is higher than in those without infections (p = 0.048). Patients with bacterial complications have an increased risk of developing more severe ACLF — grades 2 and 3 (odds ratio = 8.2; p = 0.045).
In conclusion, our study results suggest that the CLIF-C score had an advantage over the Child-Pugh score (p = 0.012 and p = 0.015, respectively) for predicting in-hospital mortality in the general group of cirrhotic patients and those with cirrhosis and infectious complications. In addition, our study demonstrates the applicability of the CLIF-C score in our settings.
Received 03.11.2021
Revised 16.11.2021
Accepted 22.11.2021
/33.jpg)

/34.jpg)
/35.jpg)
/35_2.jpg)