Журнал "Гастроэнтерология" Том 54, №4, 2020
Вернуться к номеру
Gut bacterial bile salt hydrolase activity correlates with cardiovascular risk: a case-control study
Авторы: A. Neverovskyi, V. Chernyavskyi, V. Shypulin, L. Gvozdecka, N. Mikhnova
Bogomolets National Medical University, Kyiv, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
Актуальність. Рівень сироваткового холестерину частково регулюється завдяки метаболізму жовчних кислот у кишечнику, що залежить від активності бактеріальної гідролази солей жовчних кислот (ГСЖК). Існуючі дані щодо чіткого впливу ГСЖК на ліпідний обмін та серцево-судинний ризик є недостатніми та суперечливими. Метою дослідження було оцінити взаємозв’язок між відносною активністю (ВА) кишкової бактеріальної ГСЖК і рівнями сироваткового холестерину та серцево-судинним ризиком (ССР). Матеріали та методи. Дослідження проводилось за дизайном «випадок — контроль» і включало 26 відносно здорових учасників (контрольна група) та 77 пацієнтів із дисліпідемією, без анамнезу тяжких серцево-судинних подій (основна група). В учасників були визначені загальна ВА кишкової бактеріальної ГСЖК, показники ліпідного профілю та рівні ССР за 5 шкалами ризику. Результати. ВА кишкової бактеріальної ГСЖК була вищою у здорових учасників порівняно з учасниками з дисліпідемією (р < 0,001). Було виявлено негативний кореляційний зв’язок помірної сили між ВА ГСЖК та загальним холестерином (ЗХ) (–0,38) і ліпопротеїнами низької щільності (ЛПНЩ) (–0,36) з лінійним співвідношенням, яке визначалось рівнянням: ЛПНЩ = –5,33 · ВА ГСЖК + 4,479. Було виявлено, що зі збільшенням ВА ГСЖК ризик дисліпідемії знижується (р < 0,001), ВШ = 1,06 · 10–10 (95% ДІ; 2,5 · 10–15 – 4,5 · 10–6). Виявлений помірний негативний кореляційний взаємозв’язок між ВА ГСЖК та ССР, оціненим за шкалами Globorisk (–0,34), Framingham (–0,34), алгоритмом ACC/AHA 2013 (–0,32), PROCAM (–0,35) та шкалою ВООЗ (–0,34). Висновки. Загальна ВА кишкової бактеріальної ГСЖК негативно корелювала із ЗХ, ЛПНЩ, ССР, оціненим за 5 шкалами, та негативно асоціювалася з ризиком дисліпідемії.
Актуальность. Уровень сывороточного холестерина частично регулируется благодаря метаболизму желчных кислот в кишечнике, зависящему от активности бактериальной гидролазы солей желчных кислот (ГСЖК). Существующие данные относительно четкого влияния ГСЖК на липидный обмен и сердечно-сосудистый риск недостаточны и противоречивы. Целью исследования было оценить взаимосвязь между относительной активностью (ОА) кишечной бактериальной ГСЖК и уровнями сывороточного холестерина и сердечно-сосудистым риском (ССР). Материалы и методы. Исследование проводилось по дизайну «случай — контроль» и включало 26 относительно здоровых участников (контрольная группа) и 77 пациентов с дислипидемией, без анамнеза тяжелых сердечно-сосудистых событий (основная группа). У участников были определены общая ОА кишечной бактериальной ГСЖК, показатели липидного профиля и уровни ССР по 5 шкалам риска. Результаты. ОА кишечной бактериальной ГСЖК была выше у здоровых участников по сравнению с участниками с дислипидемией (р < 0,001). Была выявлена отрицательная корреляционная связь умеренной силы между ОА ГСЖК и общим холестерином (ОХ) (–0,38) и липопротеинами низкой плотности (ЛПНП) (–0,36) с линейным соотношением, которое определялось уравнением: ЛПНП = –5,33 • ОА ГСЖК + 4,479. Было обнаружено, что с увеличением ОА ГСЖК риск дислипидемии снижается (р < 0,001), ОШ = 1,06 • 10–10 (95% ДИ; 2,5 • 10–15 – 4,5 • 10–6). Выявлена умеренная отрицательная корреляционная взаимосвязь между ОА ГСЖК и ССР, оцененным по шкалам Globorisk (–0,34), Framingham (–0,34), алгоритму ACC/AHA 2013 (–0,32), PROCAM (–0,35) и шкале ВОЗ (–0,34). Выводы. Общая ОА кишечной бактериальной ГСЖК негативно коррелировала с ОХ, ЛПНП, ССР, оцененным по 5 шкалам, и негативно ассоциировалась с риском дислипидемии.
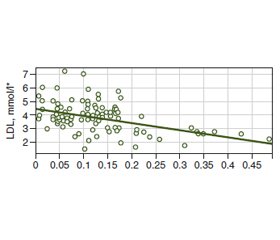
Background. Serum cholesterol may be regulated by bile acid metabolism in the gut that depends on bacterial bile salt hydrolase (BSH) activity. There are limiting data regarding the clear effect of BSH on host lipid metabolism and cardiovascular risk (CVR). The investigation aimed to assess the relationship between the gut bacterial BSH relative activity (RA) and serum cholesterol with CVR levels. Materials and methods. The investigation was conducted as a case-control study and included 26 almost healthy participants (a control group) and 77 patients with dyslipidemia and without anamnesis of major cardiovascular events (a case group). The total RA of gut BSH, lipid profile, and CVR level according to 5 risk scores were assessed. Results. The RA of BSH was higher in healthy adults comparing to participants with dyslipidemia (p < 0.001). There were found moderate negative correlation between RA of gut bacterial BSH and total cholesterol (TC) (–0.38) and moderate correlation with low-density lipoproteins (LDL) (–0.36) with linear relationship that is defined by equation: LDL = –5.33 • RA of BSH + 4.479. It was revealed that with increasing of RA of gut bacterial BSH, the risk of dyslipidemia decreased (р < 0.001), OR = 1.06 • 10–10 (95% confidence interval; 2.5 • 10–15 – 4.5 • 10–6). There was found a moderate negative correlation between RA of gut bacterial BSH and CVR levels according to Globorisk score (–0.34), Framingham score (–0.34), 2013 ACC/AHA algorithm (–0.32), PROCAM score (–0.35), and WHO risk chart (–0.34). Conclusions. The total RA of the gut bacterial BSH negatively correlated with TC, LDL, and CVR levels according to 5 risk scores and was negatively associated with the risk of dyslipidemia.
активність бактеріальної гідролази солей жовчних кислот; дисліпідемія; серцево-судинний ризик
активность бактериальной гидролазы солей желчных кислот; дислипидемия; сердечно-сосудистый риск
bile salt hydrolase activity; dyslipidemia; cardiovascular risk
Introduction
Materials and methods
Results
/29_4.jpg)
/30.jpg)
/30_2.jpg)
Discussion
Conclusions
- Mensah G.A., Roth G.A., Fuster V. The Global Burden of Cardiovascular Diseases and Risk Factors. J. Am. Coll. Cardiol. 2019. 74 (20). 2529-2532. doi: 10.1016/j.jacc.2019.10.009.
- The WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob. Health. 2019. 7. e1332-45. doi: 10.1016/S2214-109X(19)30318-3.
- Mach F., Baigent C., Catapano A.L. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020. 41 (1). 111-188. doi: 10.1093/eurheartj/ehz455.
- Ference B.A., Ginsberg H.N., Graham I. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. European Heart Journal. 2017. 38 (32). 2459-2472. doi: 10.1093/eurheartj/ehx144.
- Luo J., Yang H., Song B. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020. 21. 225-245. doi: 10.1038/s41580-019-0190-7.
- Charach G., Argov O., Geiger K., Charach L., Rogowski O., Grosskopf I. Diminished bile acids excretion is a risk factor for coronary artery disease: 20-year follow up and long-term outcome. Therap. Adv. Gastroenterol. 2018. 11. 1-11. doi: 10.1177/1756283X17743420.
- Chiang J.Y. Bile acids: regulation of synthesis. J. Lipid Res. 2009. 50. 1955-1966. doi: 10.1194/jlr.R900010-JLR200.
- Reis S.A., Conceição L.L., Rosa D.D., Siqueira N.P., Peluzio M.C.G. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutr. Res. Rev. 2017. 30 (1). 36-49. doi: 10.1017/S0954422416000226.
- Urdaneta V., Casadesús J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front. Med. (Lausanne). 2017. 4. 163. doi: 10.3389/fmed.2017.00163.
- Lau K., Srivatsav V., Rizwan A. et al. Bridging the gap between gut microbial dysbiosis and cardiovascular diseases. Nutrients. 2017. 9 (8). 859. doi: 10.3390/nu9080859.
- Geng W., Lin J. Bacterial bile salt hydrolase: an intestinal microbiome target for enhanced animal health. Anim. Health Res. Rev. 2016. 17 (2). 148-158. doi: 10.1017/S1466252316000153.
- Song Z., Cai Y., Lao X. et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome. 2019. 7. 9. doi: 10.1186/s40168-019-0628-3.
- Huijghebaert S.M., Hofmann A.F. Influence of the amino acid moiety on deconjugation of bile acid amidates by cholylglycine hydrolase or human fecal cultures. J. Lipid Res. 1986. 27 (7). 742-52. PMID: 2876046.
- Joyce S.A., MacSharry J., Casey P.G. et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl Acad. Sci. USA. 2014. 111 (20). 7421-7426. doi: 10.1073/pnas.1323599111.
- Hajifathalian K., Ueda P., Lu Y. et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes and Endocrinology. 2015. 3 (5). 339-55. doi: 10.1016/S2213-8587(15)00081-9.
- D’Agostino R.B. Sr, Vasan R.S., Pencina M.J. et. al. General Cardiovascular Risk Profile for Use in Primary Care. The Framingham Heart Study. Circulation. 2008. 117 (6). 743-53. doi: 10.1161/CIRCULATIONAHA.107.699579.
- Goff D.C. Jr, Lloyd-Jones D.M., Bennett G. et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014. 63 (2500). 2935-2959. doi: 10.1016/j.jacc.2013.11.005.
- Assmann G., Cullen P., Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation. 2002. 105 (3). 310-5. doi: 10.1161/hc0302.102575.
- Guo C.F., Li J.Y. Hypocholesterolaemic action of Lactobacillus casei F0822 in rats fed a cholesterol-enriched diet. International Dairy Journal. 2013. 32 (2). 144-149. doi: 10.1016/j.idairyj.2013.04.001.
- Li H., Xu G., Shang Q. et al. Inhibition of ileal bile acid transport lowers plasma cholesterol levels by inactivating hepatic farnesoid X receptor and stimulating cholesterol 7α-hydroxylase. Metabolism. 2004. 53 (7). 927-932. doi: 10.1016/j.metabol.2004.01.017.
- Charach G., Grosskopf I., Rabinovich A., Shochat M., Weintraub M., Rabinovich P. The association of bile acid excretion and atherosclerotic coronary artery disease. Therap. Adv. Gastroenterol. 2011. 4 (2). 95-101. doi: 10.1177/1756283X10388682.

/29.jpg)
/29_3.jpg)