Журнал "Гастроэнтерология" Том 53, №3, 2019
Вернуться к номеру
Діагностична значущість жорсткості артеріальної стінки в пацієнтів iз хронічними дифузними захворюваннями печінки
Авторы: Yu.M. Stepanov, I.S. Konenko
State Institution “Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine”, Dnipro, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
Актуальність. Діагностика хронічних дифузних захворювань печінки, серед яких найпоширенішими є неалкогольна жирова хвороба печінки (НАЖХП) та хронічний гепатит, асоційований з вірусом С (ХГС), залишається актуальною не тільки для гастроентерологів, але й для сімейних лікарів. НАЖХП тісно пов’язана з ожирінням або надлишковою масою тіла, інсулінорезистентністю, атерогенною дисліпідемією та цукровим діабетом 2 типу, частота яких на сьогодні сягає рiвня епідемії. Жирова дистрофія печінки, як неалкогольна, так і внаслідок жирового переродження під дією вірусу гепатиту С, сама по собі може бути причиною так званої печінкової інсулінорезистентності. Останніми роками був продемонстрований тісний зв’язок між стеатогепатитом та станом артеріальних судин. Так, показано, що інсулінорезистентність призводить до атеросклеротичних змін в останніх унаслідок дисліпопротеїнемії. Діаметр судин змінюється, збільшуються фрагментація еластину та відкладання колагену в стінці, що призводить до підвищення артеріальної жорсткості, в результаті чого змінюються пружноеластичні властивості артерій. Відомо, що прогресування фіброзу змінює морфологію печінки та впливає на її внутрішній та зовнішній кровообіг. Мета дослідження: визначити особливості показників локальної жорсткості артеріальної стінки в пацієнтів iз НАЖХП i ХГС та зв’язок цих показників iз фіброзом печінки. Матеріали та методи. Обстежено 195 пацієнтів, які знаходилися на обстеженні у відділенні захворювань печінки та підшлункової залози ДУ «Інститут гастроентерології НАМН України». Їх розподіляли по групах залежно від етіологічного фактора: за допомогою імуноферментного аналізу в 94 пацієнтів iз ХГС підтверджено вірусну етіологію хвороби, у 101 людини на підставі об’єктивного обстеження, інсулінорезистентності та гіперліпідемії діагностовано НАЖХП. Соноеластографію та оцінку локальної жорсткості артеріальної стінки виконували на сканері «Soneus P7» (Україна). Результати. В усіх хворих виявлено зміни показників судинної жорсткості, а саме модуля еластичності, зростання індексу артеріальної жорсткості, швидкості пульсової хвилі та товщини комплексу інтима-медіа (p < 0,05). Збільшення швидкості пульсової хвилі виявлено в 79 (78,2 %) пацієнтів iз НАЖХП (p < 0,05) і у 87 (92,5 %) обстежених iз ХГС. Медіана швидкості пульсової хвилі в пацієнтів iз НАЖХП була вищою порівняно з хворими на ХГС: 9,52 і 6,34 см/с, відповідно. У пацієнтів iз НАЖХП встановлено зв’язок жорсткості паренхіми печінки зі швидкістю пульсової хвилі (r = 0,68; p < 0,05). У хворих на ХГС виявлено асоціацію між жорсткістю печінки і товщиною комплексу інтима-медіа (r = 0,84; p < 0,001). За даними зсувнохвильової еластографії швидкість розповсюдження зсувної хвилі була більшою в пацієнтів iз захворюванням печінки вірусної етіології — 1,60 (1,44–1,94) см/с проти 1,41 (1,31–1,54) см/с у пацієнтів iз НАЖХП (p < 0,05). Медіана жорсткості печінки була вищою у хворих на ХГС — 7,77 (6,50–10,11) кПа проти 6,78 (5,49–6,90) кПа в пацієнтів iз НАЖХП (p < 0,05). Висновки. Незалежно від етіологічного чинника, при хронічних дифузних захворюваннях печінки відзначається підвищення модуля еластичності артеріальної стінки. Для хворих на ХГС поряд зі збільшенням жорсткості паренхіми печінки найбільш характерним є підвищення індексу жорсткості стінки сонної артерії. При НАЖХП відзначається зменшення розтяжності судинних стінок, що закономірно призводить до збільшення товщини комплексу інтима-медіа та швидкості пульсової хвилі.
Актуальность. Диагностика хронических диффузных заболеваний печени, среди которых наиболее распространены неалкогольная жировая болезнь печени (НАЖБП) и хронический гепатит, ассоциированный с вирусом С (ХГС), остается актуальной не только для гастроэнтерологов, но и для семейных врачей. НАЖБП тесно связана с ожирением или избыточной массой тела, инсулинорезистентностью, атерогенной дислипидемией и сахарным диабетом 2 типа, частота которых на сегодняшний день достигает уровня эпидемии. Жировая дистрофия печени, как неалкогольная, так и вследствие жирового перерождения под действием вируса гепатита С, сама по себе может быть причиной так называемой печеночной инсулинорезистентности. В последние годы была продемонстрирована тесная связь между стеатогепатитом и состоянием артериальных сосудов. Так, показано, что инсулинорезистентность приводит к атеросклеротическим изменениям в последних вследствие дислипопротеинемии. Диаметр сосудов изменяется, увеличиваются фрагментация эластина и отложение коллагена в стенке, что приводит к повышению артериальной жесткости, в результате чего изменяются упругоэластические свойства артерий. Известно, что прогрессирование фиброза изменяет морфологию печени и влияет на ее внутреннее и внешнее кровообращение. Цель исследования: определить особенности показателей локальной жесткости артериальной стенки у пациентов с НАЖБП и ХГС и связь этих показателей с фиброзом печени. Материалы и методы. Обследовано 195 пациентов, находившихся на обследовании в отделении заболеваний печени и поджелудочной железы ГУ «Институт гастроэнтерологии НАМН Украины». Их распределяли по группам в зависимости от этиологического фактора: с помощью иммуноферментного анализа у 94 пациентов с ХГС подтверждена вирусная этиология болезни, у 101 человека на основании объективного обследования, инсулинорезистентности и гиперлипидемии диагностирована НАЖБП. Соноэластографию печени и оценку локальной жесткости артериальной стенки выполняли на сканере «Soneus P7» (Украина). Результаты. У всех больных выявлены изменения показателей сосудистой жесткости, а именно модуля эластичности, увеличение индекса артериальной жесткости, скорости пульсовой волны и толщины комплекса интима-медиа (р < 0,05). Повышение скорости пульсовой волны выявлено у 79 (78,2 %) пациентов с НАЖБП (р < 0,05) и у 87 (92,5 %) обследованных с ХГС. Медиана скорости пульсовой волны у пациентов с НАЖБП была выше по сравнению с больными ХГС: 9,52 и 6,34 см/с соответственно. У пациентов с НАЖБП установлена связь жесткости паренхимы печени со скоростью пульсовой волны (r = 0,68; p < 0,05). У больных ХГС выявлена ассоциация между жесткостью печени и толщиной комплекса интима-медиа (r = 0,84; p < 0,001). По данным сдвиговолновой эластографии скорость распространения сдвиговой волны была выше у пациентов с заболеванием печени вирусной этиологии — 1,60 (1,44–1,94) см/с против 1,41 (1,31–1,54) см/с у пациентов с НАЖБП (р < 0,05). Медиана жесткости печени были выше у больных ХГС — 7,77 (6,50–10,11) кПа против 6,78 (5,49–6,90) кПа у пациентов с НАЖБП (р < 0,05). Выводы. Независимо от этиологического фактора, при хронических диффузных заболеваниях печени отмечается повышение модуля эластичности артериальной стенки. Для больных ХГС наряду с увеличением жесткости паренхимы печени наиболее характерно повышение жесткости стенки сонной артерии. При НАЖБП отмечается уменьшение растяжимости сосудистых стенок, что закономерно приводит к увеличению толщины комплекса интима-медиа и скорости пульсовой волны.
Background. Diagnosis of chronic diffuse liver diseases, among which the most common ones are nonalcoholic fatty liver disease (NAFLD) and chronic hepatitis associated with hepatitis C virus, remains topical issue not only for gastroenterologists, but also for family physicians. NAFLD is closely associated with obesity or excessive body weight, insulin resistance, atherogenic dyslipidemia, and type 2 diabetes, the frequency of which now reaches the level of epidemic. Fatty liver dystrophy, both nonalcoholic and as a result of fatty degeneration under the influence of hepatitis C virus, can itself be the cause of so-called hepatic insulin resistance. In recent years, the close link between steatohepatitis and the state of arterial vessels has been demonstrated. Thus, it is shown that insulin resistance leads to atherosclerotic changes in the latter due to the development of dyslipoproteinemia. The diameter of vessels changes, elastin fragmentation and collagen deposition in the wall increase, which leads to an increase in arterial stiffness, resulting in changed elastic properties of the arteries. Obviously, the progression of fibrosis changes liver morphology and influences its internal and external blood flow. Purpose of the study: to identify the distinguishing features of local arterial wall stiffness in patients with NAFLD and hepatitis C and correlation of these indicators with liver fibrosis. Materials and methods. One hundred and ninety-five patients were examined, they were treated at the Department of the Liver and Pancreatic Diseases of the Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine. Patients were divided into groups depending on the causative agent: viral etiology of illness was confirmed by enzyme immunoassay in 94 persons with chronic hepatitis C, and 101 patients were diagnosed with non-alcoholic fatty liver disease on the basis of objective examination, insulin resistance, and hyperlipidemia. Sonoelastography of the liver and evaluation of local arterial wall stiffness were performed on Soneus P7 scanner (Ukraine). Results. All patients had changes in the parameters of vascular stiffness, namely, in the elasticity modulus, an increase in arterial stiffness index, pulse wave velocity and intima-media thickness (p < 0.05). An increase in pulse wave velocity was detected in 79 (78.2 %) persons (p < 0.05) with NAFLD and in 87 patients (92.5 %) with hepatitis C. The median pulse wave velocity in patients with NAFLD was higher compared to those with hepatitis C: 9.52 and 6.34 m/s, respectively. In persons with NAFLD, a correlation was established between the stiffness of the liver parenchyma and pulse wave velocity (r = 0.68; p < 0.05). In patients with hepatitis C, the association was found between the liver stiffness and intima-media thickness (r = 0.84; p < 0.001). According to the shear wave elastography, shear wave velocity was higher in patients with viral hepatitis — 1.60 (1.44–1.94) m/s vs 1.41 (1.31–1.54) m/s in NAFLD patients (p < 0.05). The median liver stiffness was higher in patients with hepatitis C — 7.77 (6.50–10.11) kPa vs 6.78 (5.49–6.90) kPa in patients with NAFLD (p < 0.05). Conclusions. Regardless of the causative agent, there is an increase in the modulus of arterial wall elasticity in chronic diffuse liver diseases. For patients with hepatitis C, along with increased stiffness of the liver parenchyma, an increase in the carotid artery wall stiffness is most characteristic. The patients with NAFLD have a decrease in distensibility of vascular walls, which naturally leads to an increase in the intima-media thickness and pulse wave velocity.
артеріальна жорсткість; товщина комплексу інтима-медіа; дифузні захворювання печінки
артериальная жесткость; толщина комплекса интима-медиа; диффузные заболевания печени
arterial stiffness; carotid intima-media thickness; diffuse liver diseases
Introduction
Diagnosis and treatment of chronic diffuse liver diseases, among which the most common ones are non-alcoholic fatty liver disease (NAFLD) and chronic hepatitis associated with the C virus, remain topical issues not only for gastroenterologists, but also for family physicians. It is known that hepatits C and NAFLD are diseases, in which the pathological process is not limited only by the liver, they are characterized by the involvement of various organs and systems. General practitioners often face liver pathology in patients undergoing a survey for cardiovascular and endocrine diseases. After all, it is known that the combination of ischemic heart disease and liver disease pathogenically cause the course and progression of each other, which, in turn, significantly increases cardiovascular mortality [1]. For example, it is found that in patients with NAFLD, the incidence of cardiovascular diseases is significantly higher than in persons without NAFLD even regardless of obesity and conventional risk factors for cardiovascular pathology [2].
NAFLD is closely associated with obesity or excessive body weight, insulin resistance (IR), atherogenic dyslipidemia, and type 2 diabetes, the frequency of which to date reaches the level of epidemic [3, 4]. Fatty liver dystrophy, both non-alcoholic and as a result of fatty degeneration under the influence of hepatitis C virus, can itself be the cause of the so-called hepatic IR. In other words, IR can not only lead to liver steatosis, but also steatosis can cause IR. Insulin signals in the liver act through its receptors tyrosine kinase. Because of them, there is a negative control of glucose production by the liver, replenishment of glycogen stores and fatty acid synthesis. The development of IR leads to the suppression of these signals and increased export of triglycerides in the form of very low density lipoproteins. Furthermore, an increase in triglyceride synthesis occurs also because of elevation of lipogenesis due to the direct activation of transcription factor. The development of carbohydrate metabolism disorders is caused by the fact that the processes of gluconeogenesis are activated by hepatic IR, the production of glucose in liver is increased, and the result of these processes is hyperglycemia. Meta-analysis (2011) showed that increased overall mortality in 57.0 % of NAFLD patients was not only associated with liver disease, but also was a result of cardiovascular disease [5]. Several previous studies [6, 7] have shown that hepatitis C infection can alter carbohydrate and lipid metabolism, which in turn leads to liver resistance to peripheral insulin action and, ultimately, to the development of diabetes. The exact mechanisms underlying the development of IR, mediated by the virus, are still insufficiently studied, although several hypotheses have been proposed. One of them is inhibition, under the influence of virus, of the insulin signaling pathway by blocking the protein synthesis of the insulin receptor-1 [8].
Compensatory liver capabilities determine the fact that violation of its function is usually diagnosed already in the late stages, when most parenchyma is already replaced by the connective tissue, but there are a number of studies that indicate impaired portal blood flow already in the early stages of the chronic pathological process in the liver, which often preceding changes in the functional state of the organ.
In recent years, the close relationship between IR, steatohepatitis and the state of arterial vessels has been demonstrated. Thus, it is shown that IR leads to atherosclerotic changes in the latter due to the development of dyslipoproteinemia. The diameter of the vessels changes, elastin fragmentation and collagen deposition in the wall increase, which leads to an increase in arterial stiffness, resulting in changed elastic properties of the arteries. Evidently, the progression of hepatic fibrosis changes liver morphology and affects its internal and external blood circulation. It has been proved that the violation of hepatoportal blood circulation triggers a cascade of vegetative, neurohumoral and metabolic reactions, which cause changes in central hemodynamics, increase the disorder of intrahepatic blood circulation, in this way, completing the vicious cycle [9].
The gold standard for measuring and classifying fibrosis is liver biopsy, but due to invasive procedure and significant complications, the clinical use of this technique is limited. Therefore, today in the modern research centers, minimally invasive diagnosis dominates over invasive methods. The evaluation of haemodynamic changes does not hold aloof. Thus, with the help of new modern methods of research, it is possible to define three types of arterial stiffness: local, regional and systemic [10]. The most commonly used methods for measuring arterial stiffness include not only blood flow velocity. Measuring pulse wave velocity (PWV) is the gold standard for determining arterial stiffness. Other methods, such as measurement of central systolic pressure, augmentation index (AI), are under the influence of pathophysiologic states, medications, heart rate that makes them less reliable [11]. Some researches have been found that vascular wall elasticity is an important indicator of abnormal lipid metabolism in vascular diseases. In recent times, the analysis of vascular stiffness indicators is not limited only to cardiovascular pathology, but it is used as nonspecific marker of fibrosis [12, 13]. Thus, Japanese scientists have conducted the study in 3,040 healthy subjects measuring the serum NT-pro-BNP level as a marker of heart failure, FIB-4 scale indicators as a marker of fibrosis, elasticity of brachial artery by means of pulse wave velocity and augmentation index analysis. In 2,135 subjects, indicators were re-measured after 3 years. According to the statistical analysis, the authors concluded that there was a correlation between the degree of fibrosis and early indicators of heart failure. However, this was not associated with the vascular lesions [14].
In recent years, elastography, a new non-invasive method for assessing the degree of liver fibrosis, is widely used in clinical practice and included in manuals for the diagnosis and treatment of liver diseases. Radiofrequency signals in quantitative ultrasound vascular examination help determine intima-media thickness (IMT) and arterial stiffness and can serve as a sensitive indicator of early changes in vascular stiffness. There are studies showing that parameters of the carotid artery elasticity in patients with ischemic heart disease and diabetes differ from those of healthy people. Above, we indicated the relationship between fatty liver dystrophy and the changes of blood vessels, accompanying IR. However, rare works consider whether these parameters correlate with the degree of liver fibrosis in patients with hepatitis C and NAFLD.
Purpose of the study: to identify the distinguishing features of local arterial wall stiffness in patients with NAFLD and hepatitis C and correlation of these indicators with liver fibrosis.
Materials and methods
One hundred and ninety-five patients were examined, they were treated at the Department of the Liver and Pancreatic Diseases of the Institute of Gastroenterology of NAMS of Ukraine. Patients were divided into groups depending on the causative agent: viral etiology of illness was confirmed by enzyme immunoassay in 94 patients with chronic hepatitis C, and 101 persons were diagnosed with NAFLD on the basis of objective examination, insulin resistance, and hyperlipidemia. Morphologic verification of liver fibrosis was performed in 66 patients (33.8 %): 42 (21.5 %) — with hepatitis C and 24 (12.3 %) — with NAFLD. Among 94 persons diagnosed with hepatitis C, there were 50 (53.2 %) men and 44 (46.8 %) women, whose average age was (44.7 ± 2.3) years. Among 101 patients diagnosed with NAFLD, these indicators were as follows: 32 (31.7 %) men, 69 (68.3 %) women, (48.4 ± 2.9) years. The control group included 20 apparently healthy individuals. All patients and the control group agreed to participate in the study. Sonoelastography of the liver and evaluation of local arterial wall stiffness were performed on Soneus P7 scanner (Ukraine). The local arterial stiffness was determined by a linear sensor at a frequency of 5–12 MHz in duplex scanning. The stiffness of the vascular wall was evaluated 1.5 cm proximal to the carotid bifurcation. To assess the elastic properties of the vascular wall, measurements of blood vessels during the cardiac cycle were used. The method is realized in the form of special E-AS mode, in which the following parameters are measured automatically: minimum diameter of the vessel for a cardiac cycle (Dmin), сircumferential arterial strain (CAS), arterial stiffness index (SI), elasticity modulus (EM), AI, PWV. For measurement of intima-media thickness, E-BMI mode was used. Statistical processing of research results was carried out by means of analysis of variance using Statistica 6.0 program. The median (Me) and interquartile range (LQ — low quartiles; UQ — upper quartiles) were calculated. Evaluation of reliability of differences between the groups was carried out by means of non-parametric Wilcoxon-Mann-Whitney test. Differences were considered statistically significant if the error was less than 5 % (p < 0.05). In order to determine the interconnection between indicators, a correlation analysis was conducted with the calculation of the Spearman’s rank-order correlation coefficient (r).
Results
The changes of vascular stiffness, namely in the elasticity modulus, an increase in arterial stiffness index, pulse wave velocity and intima-media thickness (p < 0.05), were found in all patients. An increase in pulse wave velocity was detected in 79 (78.2 %) persons (p < 0.05) with NAFLD and in 87 (92.5 %) — hepatitis C.
Analysis of the obtained data revealed that median values of a number of sonographic indicators of the right and left carotid artery walls were different in patients with hepatitis C and NAFLD compared to the control group (Tables 1, 2). In our study, this applies to the left carotid artery wall stiffness due to the lower compression on a vessel with the prevailing right-handedness of the operator, through the small weight of transducer.
Thus, the average IMT increased in 100 % of patients with hepatitis C, and in 86 (85.1 %) — with NAFLD. Changes of carotid artery diameter were observed in one third of all surveyed persons (30 (30.1 %)).
Significant changes in patients with diffuse liver disease compared to the control group were observed for such ultrasonic parameters as elasticity modulus, arterial stiffness index, pulse wave velocity, and intima-media thickness. In addition, features are set depending on the causative agent, too. Thus, for patients with hepatitis C, the most characteristic is 2-fold increase in arterial stiffness index (p < 0.05) compared to the control group, and 1.5-fold increase of this index compared to those with NAFLD (p < 0.05) (Fig. 1).
For patients with liver steatosis of non-alcoholic origin only, the highest median value of the pulse wave velocity is 9.52 m/s, which is 1.7 times higher than the values in the control group — 5.57 m/s (p < 0.05), and 1.5 times higher compared to patients with hepatitis C — 6.34 m/s (p < 0.05) (Fig. 2).
The results of correlation analysis in patients with NAFLD showed an association between stiffness of the liver parenchyma and pulse wave velocity of the left carotid artery (r = 0.68; p < 0.05) according to shear wave elastography that allows us to consider this indicator a diagnostic criterion for early manifestations of atherosclerotic plaques in liver steatosis (Fig. 3).
Carotid intima-media thickening is known to be an early marker of systemic atherosclerosis and an independent risk factor for myocardial infarction, stroke, and sudden death [1]. In our study, IMT values were highest in the group of patients with NAFLD, being 0.72 mm, which is significantly higher compared to the control group — 0.52 mm (p < 0.05) and patients with hepatitis C — 0.65 mm (p < 0.05) (Fig. 4).
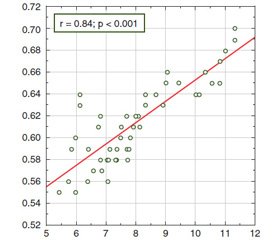
The feasibility of using IMT as a diagnostic criterion for the early detection of atherosclerosis in patients with chronic hepatitis C confirms the established correlation of such parameter of the liver parenchyma as the Young’s modulus with intima-media thickness of the left carotid artery wall (r = 0.84; p < 0.001) (Fig. 5)
The sonographical assessment of the local stiffness of the arterial wall using E-AS mode allows us to measure parameters automatically and to form an online inspection protocol (Fig. 6).
As is known, pulse wave velocity depends on the blood viscosity, heart rate and blood pressure. Its average value in patients with hepatitis C is higher compared to persons with NAFLD: 8.26 and 8.11 m/s, respectively.
Thus, a probable increase in pulse wave velocity was detected in all surveyed groups compared to the controls (p < 0.05), with the average value of this indicator in patients with hepatitis C exceeded 8.26 mm, which is 1.2 times higher than that of patients with NAFLD (p < 0.05).
To determine the intima-media thickness, carotid ultrasound was carried out to obtain a steady image of the near and far vascular walls. In the work, the far wall was examined and the following parameters were assessed: control volume width, average IMT, deviation, median value, interquartile range, minimum and maximum value (Fig. 7).
Thus, in patients of all groups a probable increase was noted compared to controls (p < 0.05), with the average IMT in patients with chronic hepatitis C exceeded 0.52 mm, which is 1.2 times higher than in the group with NAFLD (p < 0.05).
It should be noted that according to shear wave elastography, shear wave velocity was higher in patients with viral etiology of liver disease — 1.60 (1.44–1.94) m/s versus 1.41 (1.31–1.54) m/s in NAFLD patients (p < 0.05). Young’s modulus was also higher in patients with hepatitis C and amounted to 7.77 (6.50–10.11) kPa against 6.78 (5.49–6.90) kPa in patients with NAFLD (p < 0.05).
The median pulse wave velocity in patients with hepatitis C was higher compared to those with NAFLD: 9.52 and 6.34 m/s, respectively. In patients with NAFLD, a correlation was found between the stiffness of the liver parenchyma and pulse wave velocity (r = 0.68; p < 0.05). The association is established between the liver stiffness and intima-media thickness (r = 0.84; p < 0.001) in patients with hepatitis C. According to shear wave elastography, shear wave velocity was higher in patients with viral hepatitis — 1.60 (1.44–1.94) m/s vs 1.41 (1.31–1.54) m/s in NAFLD patients (p < 0.05). The median liver stiffness was higher in patients with hepatitis C — 7.77 (6.50–10.11) kPa vs 6.78 (5.49–6.90) kPa in patients with NAFLD (p < 0.05).
Discussion
Arterial stiffness depends on structural and geometric properties of the arterial wall and its ability to stretch. Among the main determinants, there are age and blood pressure. Stiffness is also a consequence of a number of interactions between different stable and dynamic parameters, for example, the state of hemodynamics, and other factors such as hormones, electrolytes and glucose [15, 16]. In our work, we have shown a correlation between the changes of liver stiffness and structural manifestations of atherosclerosis in patients with hepatitis C and NAFLD. Thus, regardless of the causative agent, elasticity modulus of blood vessels in chronic diffuse liver disease increases. This corresponds with the data of previous studies. In recent times, the amount of evidence about NAFLD association with vascular atherosclerosis, regardless of other factors of cardiovascular risk, is increasing. The risk of heart failure dependence on the presence of pronounced liver fibrosis is shown by K.A. So-Armah, J.K. Lim et al. They examined 96,373 patients over 6.9 years, including those infected with human immune deficiency virus (HIV) and hepatitis C virus. The analysis of the results showed that the degree of liver fibrosis impacts the development of heart failure, while the presence of pronounced fibrosis or liver cirrhosis is important, regardless of HIV/hepatitis C status [17]. G. Novo, F. Macaione et al. investigated signs of cardiovascular lesion, namely, stiffness of the carotid artery wall and myocardial deformation index in 39 patients with compensated cirrhosis associated with hepatitis C virus before and after treatment with direct antiviral agents. Patients examined more often demonstrated subclinical disorders of the cardiovascular system compared to the control group. The fact that the indicators improved after the eradication of the virus suggests a virus role in the occurrence and aggravation of cardiovascular pathology in this category of patients [13].
Our study has shown that NAFLD is also associated with vascular lesions and increased arterial stiffness. These findings correspond with the data of other scientists [18, 19]. In 2006, Targher et al. demonstrated that patients with steatohepatitis compared to those with simple steatosis and control group have greater carotid artery intima-media thickness. Moreover, in the same study, the degree of histological inflammation in steatohepatitis was associated with IMT, regardless of the conventional factors of cardiovascular risk, IR, and other risk factors of metabolic syndrome [20]. Marked fibrosis according to the NAFLD fibrosis score is positively associated with carotid intima-media thickness and plaques in patients with NAFLD, regardless of conventional cardiometabolic risk and IR factors [21].
Perhaps, a common link between liver fibrosis and vascular atherosclerosis is the presence of systemic inflammation, which may lead to endothelial deformation, particularly in liver sinusoids and arterial wall. K. Ozturk, O. Kurt et al. showed that fibrosis in patients with NAFLD, regardless of the components of metabolic syndrome, is accompanied by an increase in pentraxin-3 serum level, which is a marker of inflammation in the cardiovascular system. The close association between level of this protein and the arterial stiffness in patients with hepatic dystrophy [22] was demonstrated for the first time in this study. In 2018, correlation between histological activity of inflammation, arterial stiffness, and endothelial dysfunction in NAFLD has been confirmed by work of A. Tuttolomondo, S. Petta et al., when examining 80 patients with steatosis and 83 healthy controls [23].
Turkish scientists investigated the relationship between the degree of vascular and hepatic stiffness in 125 patients with morphologically proven NAFLD. The study was conducted using the Mobil-O-Graph monitor on the brachial artery. Arterial elasticity was evaluated by analyzing pulse wave velocity and augmentation index. The indicators were higher than the data of the control group patients. Liver stiffness weakly, but reliably correlated with both PWV and AI [12]. In our study, the correlation between PWV and carotid artery stiffness was even more close (r = 0.68; p < 0.05). According to M. Sunbul, M. Agirbasli et al., an increase in arterial stiffness (data from Mobil-O-Graph arteriography) correlated with severity of hepatic fibrosis and increased thickness of epicardial fat in NAFLD patients [24].
We investigated carotid artery stiffness using another method, but our data largely correspond with the data of previous researchers.
Conclusions
1. Regardless of the causative agent in chronic diffuse liver diseases, there is an increase in the modulus of the arterial wall elasticity.
2. For patients with hepatitis C, along with increased stiffness of the liver parenchyma, an increase in the carotid artery wall stiffness is most characteristic.
3. The patients with NAFLD have a decrease in distensibility of vascular walls, which naturally leads to an increase in the intima-media thickness and pulse wave velocity.
Conflicts of interests. Authors declare the absence of any conflicts of interests and their own financial interest that might be construed to influence the results or interpretation of their manuscript.
1. Myhajlovs’ka N.S., Minjajlenko L.Je. Relationship of non-alcoholic fatty liver disease with components of metabolic syndrome in patients with coronary heart disease. Bukovina Medical Bulletin. 2016. 1(77). 79-83 (in Ukrainian).
2. Vakaljuk I.I. Influence of non-alcoholic liver disease on the effectiveness of antitrombocitary therapy in patients with stable coronary heart disease after revascularization interventions. 2018 Feb. 2(20). https://ws-conference.com/webofscholar.
3. World Gastroenterology Organization. Obesity: World Gastroenterology Organization Global Guideline. World Gastroenterology Organization. 2012. Cited 2012, Dec 1. 3-17. https://www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics.
4. Stepanov Ju.M. Hepatic steatosis and steatohepatitis is the inevitability of mixed genesis. Gastroenterology. 2014. 4. 136-142.
5. Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011 Dec. 43(8). 617-49.
6. Banerjee S., Saito K., Ait-Goughoulte M., Meyer K., Ray R.B., Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J. Virol. 2008. 82. 2606-12.
7. Huang J.F., Yu M.L., Dai C.Y., Chuang W.L. Glucose abnormalities in hepatitis C virus infection. Kaohsiung J. Med. Sci. 2013. 29. 61-8.
8. Ting-ting Gao, Zhao-ling Qin, Hao Ren, Zhong-tian Qi. Inhibition of IRS-1 by hepatitis C virus infection leads to insulin resistance in a PTEN-dependent manner. Virol. J. 2015. 12. 12. doi: 10.1186/s12985-015-0241-4.
9. Kas’janova T.R. Hemodynamic disorders and myocardial dysfunction in chronic hepatitis and cirrhosis: abstract for doctor of medical sciences. Astrahan, 2014. 47. (in Russian).
10. Miliagin V.A., Komissarov V.B. Modern methods of evaluation of vascular stiffness. Arterial Hypertension. 2010. 16(2). 1-10. (in Russian).
11. Townsend R.R., Wilkinson I.B., Schiffrin E.L., Avolio A.P., Chirinos J.A., Cockcroft J.R. et al.; American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015. 66(3). 698-722.
12. Bilgin B.O., Sunbul M., Kani H.T., Demirtas C.O., Keklikkiran C., Yilmaz Y. Arterial stiffness is associated independently with liver stiffness in biopsy-proven nonalcoholic fatty liver disease: a transient elastography study. Eur. J. Gastroenterol. Hepatol. 2019, Jun 6. doi: 10.1097/MEG.0000000000001471.
13. Novo G., Macaione F., Giannitrapani L., Minissale M.G., Bonomo V., Indovina F. et al. Subclinical cardiovascular damage in patients with HCV cirrhosis before and after treatment with direct antiviral agents: a prospective study. Aliment. Pharmacol. Ther. 2018 Oct. 48(7). 740-749. doi: 10.1111/apt.14934. Epub 2018 Aug 10.
14. Iwasaki Y., Tomiyama H., Shiina K., Matsumoto C., Kimura K., Fujii M. et al. Liver stiffness and arterial stiffness/abnormal central hemodynamics in the early stage of heart failure. Int. J. Cardiol. Heart Vasc. 2018, Jul 23. 20. 32-37. doi: 10.1016/j.ijcha.2018.07.001. eCollection 2018 Sep.
15. Laurent S., Cockcroft J., van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D. et al. European Network for Non-invasive Investigation of large arteries. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006. 27. 2588-2605. doi: 10.1093/eurheartj/ehl254.
16. Laurent S., Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension. 2007. 49. 1202-1206. doi: 10.1161/Hypertensionaha.106.076166.
17. So-Armah K.A., Lim J.K., Lo Re V. FIB-4 stage of liver fibrosis predicts incident heart failure among HIV-infected and uninfected patients. Hepatology. 2017. 66. 1286-1295.
18. Oni E.T., Agatston A.S., Blaha M.J. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013. 230. 258-267.
19. Chen Y., Xu M., Wang T. Advanced fibrosis associates with atherosclerosis in subjects with nonalcoholic fatty liver disease. Atherosclerosis. 2015. 241. 145-150.
20. Targher G., Bertolini L., Padovani R., Rodella S., Zoppini G., Zenari L. et al. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006 Jun. 29(6). 1325-30.
21. Chen Y., Xu M., Wang T., Sun J., Sun W. et al. Advanced fibrosis associates with atherosclerosis in subjects with nonalcoholic fatty liver disease. Atherosclerosis. 2015 Jul. 241(1). 145-50. doi: 10.1016/j.atherosclerosis.2015.05.002. Epub 2015 May 6.
22. Ozturk K., Kurt O., Dogan T., Ozen A., Demirci H., Yesildal F., Kantarcioglu M. et al. Pentraxin 3 is a predictor for fibrosis and arterial stiffness in patients with nonalcoholic fatty liver disease. Gastroenterol. Res. Pract. 2016. 2016. 1417962. doi: 10.1155/2016/1417962. Epub 2016 Feb.
23. Tuttolomondo A., Petta S., Casuccio A., Maida C., Corte V.D., Daidone M., Di Raimondo D. et al. Reactive hyperemia index (RHI) and cognitive performance indexes are associated with histologic markers of liver disease in subjects with non-alcoholic fatty liver disease (NAFLD): a case control study. Cardiovasc. Diabetol. 2018, Feb 16. 17(1). 28. doi: 10.1186/s12933-018-0670-7.
24. Sunbul M., Agirbasli M., Durmus E., Kivrak T., Akin H., Aydin Y. et al. Arterial stiffness in patients with non-alcoholic fatty liver disease is related to fibrosis stage and epicardial adipose tissue thickness. Atherosclerosis. 2014 Dec. 237(2). 490-3. doi: 10.1016/journal.atherosclerosis.2014.10.004. Epub 2014 Oct 17.

/41-1.jpg)
/42-1.jpg)
/43-1.jpg)
/43-2.jpg)