Introduction
Coronavirus disease (COVID-19) which is caused by SARS-CoV-2 is a potentially fatal acute respiratory syndrome. It was announced from Wuhan, China first on December 2019 and became a world health problem in a short time and declared as a pandemic by World Health Organisation on March 2020 [1].
Cancer is one of the most common diseases and has a multidisciplinary treatment schedule. Radiotherapy is one of the most important cancer treatment methods. As reported in recent data delays in radiotherapy affects the treatment outcomes like local control and overall survival [2, 3].
COVID-19 has a higher incidence and worse prognosis on patients with cancer, which is possibly due to their immunological status or frequent hospital visits [4]. Besides higher incidence COVID-19 is associated with higher mortality rates [5].To protect oncologic patients from COVID-19 and its complications some measures were taken in radiation oncology departments like changing the workflow, using special personal protective clothing ,temperature checks in the entrance of the clinics and daily symptom questionnaires [6, 7].
Purpose. Besides all these measures some of the patients were infected with COVID-19 during the radiotherapy. This study was planned to evaluate the course of the infection in cancer patients infected with COVID-19 during radiotherapy and the effect of the interruption to radiotherapy due to infection on the effectiveness of treatment.
Materials and methods
This is a retrospective, observational, single-center study. The study included adult patients (18–85 years old) with the diagnosis of solid cancer who received radiotherapy (RT) in curative, adjuvant or neoadjuvant intent between March 2020 — May 2021. All patients had pathologically confirmed cancer diagnoses. Metastatic patients were excluded from the study. All patients receiving radiotherapy were questionnaired for COVID-19 symptoms daily and nasopharyngeal and oropharyngeal swab were performed to all patients with suspicious symptoms. The Computed Tomography (CT) chest was also performed if clinically needed. Patients with COVID-19 positive swab were included in this study. The demographic characteristics of patients, primary cancer diagnosis, the stage of cancer, the aim of radiotherapy administration (curative, adjuvant or neoadjuvant) and chemotherapy intake, interval time of radiotherapy, COVID-19 the recovery condition, and the condition of patient on the last clinic control were all collected from the clinical records of the radiation oncology department. Interval time of fourteen days for radiotherapy was considered as the cut-off value based on the recent data [8].
The study was approved by Ankara Oncology Training and Research Hospital Ethical Committee with a number of 2021-06/1156 in June 2021.
Statistical analysis
The statistical analysis was performed using SPSS (Statistical Package for Social Sciences; SPSS Inc. Chicago, IL) version 22 software package. The descriptive categorical data were expressed as n and % values while descriptive continuous data were presented as mean ± standard deviation (mean ± SD) in the study. The Chi-Square test was applied for the comparison between the groups regarding categorical variables. Log-rank (Mantel-Cox) analysis was performed to compare overall survivals between categories. The statistical significance level of the analyses was accepted as p < 0.05 value.
Results
1800 cancer patients who have received radiotherapy for curative, adjuvant or neoadjuvant intent between March 2020 — May 2021 were evaluated for this study. Thirty-four patients infected with COVID-19 disease during the radiotherapy were enrolled into study. The COVID-19 incidence in our clinic was 0.01.
Fifteen patients were male (44.1 %) and nineteen (55.9 %) were female. The mean age of the patients was 58.4 ± 12.3 (min = 19/max = 84). When The Eastern Cooperative Oncology Group Performance Status (ECOG) of the patients was examined, it was observed that nine (26.5 %) were active (ECOG 0), twenty-two (64.7 %) were limited (ECOG 1), and three (8.8 %) were symptomatic (ECOG 2). Seven (20.6 %) of the patients had diabetes mellitus (DM), fifteen (44.1 %) had hypertension (HT) and three (8.8 %) had chronic obstructive pulmonary disease (COPD), while twelve (35.3 %) were smokers. When considered the cancer stage; six (17.64 %) patients were stage I, six (17.64 %) were stage II, and twenty-two (64.7 %) were stage III. Eighteen (52.9 %) of the patients received adjuvant RT, fourteen (41.2 %) of them received curative RT, and two (5.9 %) of them received neoadjuvant RT.
The most observed symptoms related to COVID-19 were fever (n = 29,85.2 %), cough (n = 20,58.8 %), loss of appetite (n = 12,35.2 %), anosmia and/or taste loss (n = 7,20.5 %).
All patients had nasopharyngeal swap and 7 had also chest computed tomography for COVID-19 diagnosis. All nasopharyngeal swaps were positive. Lung involvement was observed on 3 of the chest scans. Sixteen patients were hospitalized for COVID-19 treatment, others were followed up at their homes. None of the patients died due to COVID-19 infection, they all had recovery and continued their cancer treatment.
The median interval time to radiotherapy was 14 days (range 10–30 days). While RT treatment of fourteen patients (41.2 %) was interrupted for less than 14 days, RT treatment of twenty (58.8 %) patients was interrupted for 14 days or more. Twenty-one (61.8 %) of the patients had a complete response at the 3rd month, while twenty (64.5 %) of them had a complete response at the 6th month. In the last case, twenty (58.8 %) patients had a complete response, one (2.9 %) had a partial response, two (5.9 %) had distant metastases, two (5.9 %) had progression, while nine (26.5 %) of them had died.
/5.jpg)
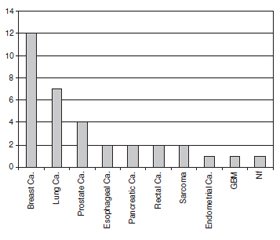
When the patients were analyzed according to their diagnosis, it was found that twelve (35.3 %) of them had breast cancer, seven (20.6 %) of them had lung cancer, four (11.8 %) of them had prostate cancer, two (5.9 %) of them had esophageal cancer, two (5.9 %) of them had pancreatic cancer, two (5.9 %) of them had rectal cancer, two (5.9 %) of them had sarcoma, one (2.9 %) of them had endometrial cancer, one (2.9 %) of them had glioblastoma multiforme (GBM), and one (2.9 %) of them had Nasopharyngeal (NF) carcinoma (Fig. 1).
64.3 % of those who interrupted RT for less than fourteen days had a complete response, 7.1 % of them had a partial response, and 28.6 % of them died. On the other hand, complete response was observed in 55 % of those who were interrupted for 14 days or more, distant metastasis in 10 % of them, progression in 10 % of them, and death in 25 % of them. There is no significant difference between RT interval times in the last control status (p = 0.537) (Table 2).
Nine of the 34 patients included in the study died and the overall survival rate was 73.5 %. When all patients were evaluated together, the mean survival time was found to be 20.3 months. The 12-month and 15-month survival rates were respectively 90.1 and 72.8 % (Figure 2).
There is a significant difference between the ECOG categories in terms of mean survival (p = 0.021). This significant difference is due to the difference between the symptomatic group and the other two stages, and the symptomatic group had a lower mean survival time than the other two stages. There is no significant difference in survival between gender (p = 0.643) and the interval between RT (p = 0.861) (Table 3).
Discussion
In this study we described both the demographic and clinical characteristics of 34 cancer patients infected with COVID-19 during their radiotherapy process. We reported the cancer treatment outcomes in terms of local recurrence and overall survival.
Bondeson et al. reported 1.3 % COVID-19 incidence in 10 774 cancer patients [9]. COVID-19 incidence was 0.01 % in our cancer patients. These low rates at our clinic may be due to strict precautions taken against COVID-19 in our hospital and clinic.
Breast cancer was the most frequent cancer type in our patient group despite the recent data reporting the lung cancer as the most frequent cancer type [8, 10]. This could have belonged to our patients’ female dominance (55.9 %).
The most frequent blood type was A Rh-positive in this study. The association between COVID-19 risk and blood type is controversial in recent studies. Kerbage et al. compared the blood type of 474 patients COVID-19 and reported that A Rh-positive group is higher in COVID-19 positive group compared with the general population (p < 0.001) [11]. Donskov et al. compared the blood groups of 12 120 COVID-19 positive patients and 118 801 healthy plasma donors, they reported that blood group A was higher in the infected group compared to uninfected healthy group (41.54 and 34.39 % respectively, p < 0.05) [12]. Despite these results Pasangha et al. reported that B blood group has the highest prevalence in COVID-19 positive patients [13].
In this study we didn’t observe any significant difference in local control or overall survival belonging to radiotherapy interval time. In a review by Ferriera et al. it was reported that there is a significant relationship between the overall treatment time and locoregional control in head and neck cancers. Loss of local control ranging between 1.2 to 12–14 % due to the radiotherapy interval time [2]. In a study by Perez et al. they reported that overall treatment time had a major impact on pelvic tumor and 10-year cause-specific survival [14]. Our sample size was small, and the patient group was heterogenous, these could be the main reasons why we could not find a relationship between local control and radiotherapy interval time. ECOG performance status and the primary cancer type were the important parameters for overall survival in our study. Better ECOG performance status was related to better overall survival. The lung cancer diagnosis was related to worse overall survival.
Conclusions
In our study there was no significant relationship between the local control and the radiotherapy interval time in COVID-19 positive cancer patients. More studies with more patient numbers could serve as informative points about this topic.
Received 24.12.2021
Revised 18.01.2022
Accepted 25.01.2022
Список литературы
1. Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020. 109. 102433. Epub 2020/03/03. doi: 10.1016/j.jaut.2020.102433. PubMed PMID: 32113704; PubMed Central PMCID: PMCPMC7127067.
2. Gonzalez Ferreira J.A., Jaen Olasolo J., Azinovic I., Jeremic B. Effect of radiotherapy delay in overall treatment time on local control and survival in head and neck cancer: Review of the literature. Rep. Pract. Oncol. Radiother. 2015. 20(5). 328-39. Epub 2015/11/10. doi: 10.1016/j.rpor.2015.05.010. PubMed PMID: 26549990; PubMed Central PMCID: PMCPMC4597087.
3. Soyfer V., Geva R., Michelson M., Inbar M., Shacham-Shmueli E., Corn B.W. The impact of overall radiotherapy treatment time and delay in initiation of radiotherapy on local control and distant metastases in gastric cancer. Radiat. Oncol. 2014. 9. 81. Epub 2014/03/25. doi: 10.1186/1748-717X-9-81. PubMed PMID: 24655942; PubMed Central PMCID: PMCPMC3994343.
4. Nowroozi A., Razi S., Sahu K.K., Grizzi F., Arends J., Keshavarz-Fathi M. et al. COVID-19 in Patients with Cancer. Adv. Exp. Med. Biol. 2021. 1318. 315-31. Epub 2021/05/12. doi: 10.1007/978-3-030-63761-3_18. PubMed PMID: 33973186.
5. Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020. 323(18). 1775-6. Epub 2020/03/24. doi: 10.1001/jama.2020.4683. PubMed PMID: 32203977.
6. van de Haar J., Hoes L.R., Coles C.E., Seamon K., Frohling S., Jager D. et al. Caring for patients with cancer in the COVID-19 era. Nat. Med. 2020. 26(5). 665-71. Epub 2020/05/15. doi: 10.1038/s41591-020-0874-8. PubMed PMID: 32405058.
7. Meattini I., Franco P., Belgioia L., Boldrini L., Botticella A., De Santis M.C. et al. Radiation therapy during the coronavirus disease 2019 (covid-19) pandemic in Italy: a view of the nation’s young oncologists. ESMO Open. 2020. 5(2). Epub 2020/04/17. doi: 10.1136/esmoopen-2020-000779. PubMed PMID: 32295769; PubMed Central PMCID: PMCPMC7199912.
8. Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R. et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020. 31(7). 894-901. Epub 2020/04/01. doi: 10.1016/j.annonc.2020.03.296. PubMed PMID: 32224151; PubMed Central PMCID: PMCPMC7270947.
9. Bondeson L., Thulin A., Ny L., Levin M., Svensson J., Lindh M. et al. Clinical outcomes in cancer patients with COVID-19 in Sweden. Acta Oncol. 2021. 1–8. Epub 2021/09/18. doi: 10.1080/0284186X.2021.1973679. PubMed PMID: 34530692.
10. Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z. et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020. 10(6). 783-91. Epub 2020/04/30. doi: 10.1158/2159-8290.CD-20-0422. PubMed PMID: 32345594; PubMed Central PMCID: PMCPMC7309152.
11. Kerbage A., Haddad S.F., Nasr L., Riachy A., Mekhael E., Nassim N. et al. Impact of ABO and Rhesus blood groups on COVID-19 susceptibility and severity: A case-control study. J. Med. Virol. 2021. Epub 2021/11/11. doi: 10.1002/jmv.27444. PubMed PMID: 34755349; PubMed Central PMCID: PMCPMC8662239.
12. Donskov S.I., Bulanov A.Y., Simarova I.B., Belyakova V.V., Mayorova O.A., Kravtsova E.A. et al. AB0 and rhesus blood groups as a risk factor for ARVI COVID-19. Klin. Lab. Diagn. 2021. 66(11). 661-5. Epub 2021/12/10. doi: 10.51620/0869-2084-2021-66-11-661-665. PubMed PMID: 34882350.
13. Pasangha E., Dhali A., D’Souza C., Umesh S. Are blood groups related to the distribution and severity of COVID-19? A cross-sectional study in a tertiary care hospital in South India. Qatar. Med. J. 2021. 2021(3). 63. Epub 2021/12/11. doi: 10.5339/qmj.2021.63. PubMed PMID: 34888199; PubMed Central PMCID: PMCPMC8627574.
14. Perez C.A., Grigsby P.W., Castro-Vita H., Lockett M.A. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1995. 32(5). 1275-88. Epub 1995/07/30. doi: 10.1016/0360-3016(95)00220-S. PubMed PMID: 7635767.

/5.jpg)
/6_2.jpg)
/6.jpg)
/7.jpg)