Introduction
Cancer is one of the most important diseases worldwide [1]. Breast cancer is one of the most common cancer types among women and ranks second in cancer-related death [2]. MCF-7 and MDA-MB-231 cell lines are used in cancer studies. It is known that the extracellular matrix plays an important role in cancer development and metastasis [3]. It is suggested that the main mechanism in the development of metastasis is the disruption of the intercellular relationships of cancer cells. Destabilization of cell adhesion molecules is considered to be the main responsible of changes in cancer cells [4].
CD44 is a member of the superfamily hyaluronan (HA) binding proteins (HABPs) or hyaladherins that play a role in cell adhesion, migration, invasion and survival. Also, it is a common marker for “tumor initiating cells-cancer stem cells” (CSC) in human carcinomas [5]. CD44 consists of three domains, the extracellular domain, the transmembrane domain and the intracellular domain. Also, CD44 has two isoforms, the standard form of CD44 (CD44s) and the variants of CD44 (CD44v). The important domain in the formation of isoforms is the extracellular domain. Different variants arise due to alternative splicing occurring in the exons of this domain [6, 7].
CD44 are several variants due to alternative splicing and these variants have important role [8]. The standard form of CD44s is widely expressed in cells of different healthy tissues. CD44v is associated inflammation and various types of cancer. Also up-regulation of CD44v promotes tumor progression and metastasis [6].
Receptor for HA-mediated motility (RHAMM) also is known as CD168. RHAMM is found in the cell, including as extracellular and intracellular. Extracellular RHAMM interacts with receptor tyrosine kinases and some non-receptor tyrosine kinases. Extracellular RHAMM can regulate cellular transformation and migration depending on HA. Intracellular RHAMM also binds to a number of proteins that can regulate microtubule dynamics and centrosome structure/function through ERK1/2/MAP kinase activation, which contributes to microtubule-mediated cell polarity and cell migration [8–10]. Nuclear RHAMM also binds to the ERK1/2/MAP kinase, which mediates the activation of PAI-1 and MMP-9, which are involved in cell motility and inflammation [8, 11]. In addition, RHAMM affects microtubules, centrosomes and mitotic spindles, while it is expressed from the thymus, testis and placenta under physiological conditions, it is especially expressed from solid tumors. Also RHAMM exhibits basal motility in cancer cell lines and its hyperexpression is considered a marker of poor prognosis [4, 12].
Rapamycin is used as an mTOR inhibitor and in the downstream pathway of mTORC1, blocks p70 S6 kinase activation [13]. The mTOR pathway plays diffrent roles of cell signaling and development [14]. It is activated in some human cancers, such as breast cancer [15]. mTORC1 is observed in numerous human cancers due to mutations in oncogenes and in tumor suppressors [16]. These mutations give cancer cells a growth advantage over normal cells [17]. To meet the needs required in proliferation, cancer cells are usually controlled by the mTORC1 pathway. Therefore, drugs targeting mTORC1 (such as rapamycin) are expected to disrupt cancer metabolism and are considered promising anti-cancer therapies [16].
In this study, we purposed the changes on the HA receptors CD44 and RHAMM after Rapamycin was administered in MCF-7 and MDA-MB-231 cell lines, which are breast cancer cells of different molecular and biological characteristics.
Materials and Methods
Cell Culture of MCF-7 and MDA-MB-231
MCF-7 (HTB-22™) and MDA-MB-231 (HTB-26™) were purchased from the American Type Culture Collection (ATCC) was used in this study. Cell lines were cultivated in RPMI-1640 medium (Capricorn, Germany) with 10% fetal bovine serum (FBS, Biochrom AG, Germany) 1% L-glutamine (Capricorn, Germany) and 1% Penicillin and Streptomycin (Biochrom AG, Germany) in 5% CO2 humidity incubator at 37 °C. The cells were passaged after reaching 80% confluency and seeded into 24-well plates on sterilized cover slips.
Drug Treatment
The IC50 dose of rapamycin (Rapamune, Pfizer) were determined as 1 μg/ml [18]. Both non-metastatic and metastatic cancer cell lines divided two group as control and rapamycin group. Control groups in all experiments were performed complete medium and rapamycin groups in all experiments were performed 1 μg/ml rapamycin in complete medium for 24 hours.
Immunocytochemical Staining
Cells were fixed with 4% paraformaldehyde for 30 minutes at room temperature. In order to inhibit the endogenous peroxidase activity, 3% H2O2 (Merck, Germany) was performed for 10 minutes at room temperature. 0.01% Triton X100 (Santa Cruz, USA) in PBS was kept on ice for 15 minutes to permeabilize the cells. Cells were blocked with the blocking solution (Invitrogen, USA) at room temperature for 1 hour. It was incubated at 4 °C overnight with CD44 (BA0321, Boster, Wuhan, China) and RHAMM (BA3380, Boster, Wuhan, China) antibodies (1 : 100 in PBS). Biotinylated secondary antibody was treated at room temperature for 30 minutes. Streptavidin was treated at room temperature for 30 minutes. The antigen antibody complex was made visible with 3,3-diaminobenzidine. The cells were counterstained Mayer’s hematoxylin. After staining cells were rinsed with deionized water and covered mounting medium. After each solution except blocking, it was washed 3 times with PBS for 5 minutes. CD44 and RHAMM expressions in the cells of each group were evaluated semiquantitatively using H-score analysis. The immunostaining intensities were categorized by the following scores: 0 (no staining), 1 (weak), 2 (moderate), and 3 (strong) [19].
qRT-PCR Analysis
Total RNA was carried out from cells using GeneMATRIX Universal RNA Purification Kit (E3598, EURx) according to the kit protocol and manufacturer’s instructions. In order to determine the expression of CD44, RHAMM and GAPDH genes, cDNA synthesis was performed immediately after total RNA isolation by using NG dART RT Kit (E0801-02, EURx). qRT-PCR process from obtained cDNAs was carried out using the EvaGreen qPCR Mix Plus (Solis BioDyne, 08-24-00001). qRT-PCR was performed in triplicate for each target gene and sample on a StepOnePlus™ Real-Time PCR System (Applied Biosystems); a negative control (ddH2O) was added for each sample. Sequences of primer pairs are shown in table 1. GAPDH gene was used as reference gene. Relative expression of target genes was evaluated according to 2−∆∆Ct method [20].
Statistical Analysis
Statistical analysis were performed via Graph Pad Prism 8.0 (GraphPad Software, San Diego, CA, USA). For H-score results, Kruskal-Wallis H test, which is a non-parametric test, performed. A value of of p < 0.001 was accepted statistically significant. For qRT-PCR results, Shapiro-Wilk test was applied. As the values showed normal distribution, H-scores and gene expressions between groups were compared using Student t-test and the means and standard deviations were determined. Р < 0.05 was considered statistically significant.
Results
Immunohistochemical Results
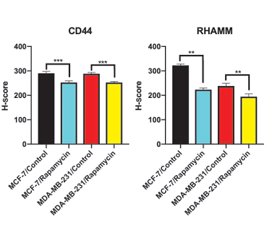
In the control group of MCF-7 cells, CD44 immunoreactivity was observed moderate in the cytoplasm and strong in dividing cells (fig. 1A). It was observed that immunoreactivity was weak in the rapamycin-treated group (fig. 1B). The H-score value of rapamycin-treated group (252.000 ± 6.346) was statistically decreased compared to the control group (290.000 ± 7.454) (p < 0.05, p < 0.0002, fig. 3, table 2).
In MDA-MB-231 cells, CD44 immunoreactivity was observed weak/moderate in control group (fig. 1C). Weak immunoreactivity was observed in the rapamycin-treated group (fig. 1D). The H-score value of rapamycin-treated group (252.500 ± 4.249) was statistically decreased compared to the control group (288.000 ± 5.375) (p < 0.0009, fig. 3, table 2).
In MCF-7 cells, RHAMM immunoreactivity was observed moderate in control group (fig. 2A). The immunoreactivity of rapamycin-treated group was weak (fig. 2B). The H-score value of rapamycin-treated group (223.000 ± 7.149) was statistically decreased compared to the control group (322.500 ± 5.401) (p < 0.0013, fig. 3, table 2).
In control group of MDA-MB-231 cells, RHAMM immunoreactivity was moderate in cytoplasm and nucleus (fig. 2C). It was observed that immunoreactivity was weak in cytoplasm but strong in nucleus in rapamycin treated group (fig. 2D). The H-score value of rapamycin-treated group (194.50 ± 11.41) was statistically decreased compared to control group (238.00 ± 11.11) (p < 0.0013, fig. 3, table 2).
qRT-PCR Results
According to the qRT-PCR results, rapamycin treatment was lead to decrease of CD44 expression in MCF-7 cells approximately 1.370 ± 0,039 fold and in MDA-MB-231 cells 1.410 ± 0,048 fold compared to control groups (p < 0.05). RHAMM expressions in MCF-7 and MDA-MB-231 cells were also decreased rapamycin treated groups compared to control groups (1.81 ± 0,13 and 1.310 ± 0,045 fold, respectively, p < 0.05, fig. 4).
Discussion
In this study, we evaluated the changes in the expression levels of CD44 and RHAMM at the 24th hour with immunocytochemical and qRT-PCR methods after rapamycin administration. We found that the expression of CD44 and RHAMM in MCF-7 and MDA-MB-231 cells decreased both immunohistochemically and by qRT-PCR. The activation of MTOR complexes will give tumors a great growth advantage with mutations, increased protein synthesis and increased inhibition of autophagy. This situation provides an advantage for cancer cells to selectively progress and proliferate compared to normal cells. mTOR expression is associated poor prognosis in breast cancer [21]. Although rapamycin is known to inhibit the selective mTORC1 inhibitor and their downstream effectors, it also indirectly inhibits mTORC2 in long-term use [22]. In our results, the reduction of CD44 and RHAMM expression in both MCF-7 and MDA-MB-231 cells at 24 hours is initially positive, but it is controversial that the same effect will be seen in rapamycin applications for 48 hours and longer. As a matter of fact, studies have shown that long-term rapamycin use causes increased expression of AKT in upstream signal activation of mTOR and increased use of mTORC2 signaling pathway [18, 22]. It suggests that this increase will only increase the expression levels of other survival pathways in the AKT activation related pathways, in addition to the greater use of the mTORC2 pathway. In order to prevent such increases, the long-term effects of rapamycin with different drugs and/or inhibitors that may affect mTORC2 and different survival pathways should be studied. CD44 is highly expressed in many cancers and regulates metastasis that is a cell surface adhesion receptor. Its interaction with appropriate extracellular matrix ligands supports the migration and invasion processes in metastasis [23]. The gene expression and protein expression of CD44 decreased in MCF-7 and MDA-MB-231 cells. The decrease in CD44 suggested that it decreased the motility of the cells in both cell lines. As a matter of fact, it has been reported that there are different variants of CD44 and that CD44v5, CD44v6, CD44v8-10 from these variants show different expression in breast cancer [6]. In addition, Afifiy et al. indicated that CD44s and CD44v6 play a role in breast cancer cell adhesion, motility and invasion through their interaction with Hyaluronic acid (HA). They reported that antibodies against different epitopes on CD44 can mediate different functional effects for breast cancer cells and be used to inhibit migration and invasion of breast cancer cells in vivo [24]. Analysis of gain and loss of function for CD44v6 in MCF-7 cells demonstrated that overexpression of CD44v6 increased the endogenous invasive capacity of activated epidermal growth factor receptor (EGFR) signaling and attenuated its response to endocrine therapy. Thus, a relationship was found between suppression of CD44v6 and decreased invasive capacity in endocrine-resistant breast cancer cells [25, 26]. Also, Bai et al. suggested that CD44/PI3K/AKT/mTOR pathway may be a treatment target in triggering apoptosis of MDA MB-231 cells [27].
RHAMM is increased in several type of cancers. It play a role in maintaining the stability of the mitotic spindle [28] and promoting cell motility and invasiveness [4]. We found that RHAMM decreased at the 24th hour of rapamycin administration in both cell lines. This result suggests that rapamycin effectively suppresses the ability of cancer cells to invade through RHAMM for a short time. Also Wang et al. showed that cell motility decreased with RHAMM knockdown in their study [29]. In another study, the mevalonate and Hippo pathways that regulate the cancer metastasis of RHAMM have been revealed and the suppression of RHAMM will also prevent this activation [30]. Schütze et al. notified that the detection of splicing variants of RHAMM in relation to the P53 mutation status is important in determining the sensitivity to radiotherapy in breast cancer cell lines [31]. This study is important in terms of invasion, cell motility and metastasis in splice variants apart from evaluating RHAMM as pan. Knowing different variants of RHAMM and their different expression in different breast cancer cell lines will cause changes in the treatments to be applied. In the light of the studies and our study, it was thought that RHAMM is an important marker in breast cancer, and suppression of RHAMM will contribute positively to the treatment to be applied.
Conclusions
As a result, our study showed that expressions of CD44 and RHAMM decreased in non-metastatic and metastatic cell lines at the 24th hour of Rapamycin administration. In addition, examining the variants of CD44 and RHAMM suggested that more effective treatment options could be created in the personalized treatment of breast cancer.
Received 28.09.2021
Revised 05.10.2021
Accepted 11.10.2021
Список литературы
1. Barzaman K., Karami J., Zarei Z., Hosseinzadeh A., Kazemi M.H., Moradi-Kalbolandi S., Safari E., Farahmand L. Breast cancer: Biology, biomarkers, and treatments. International Immunopharmacology. 2020 Jul. 84. 106535. doi: 10.1016/j.intimp.2020.106535. Epub 2020, Apr 29. PMID: 32361569.
2. Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021 May. 71(3). 209-249. doi: 10.3322/caac.21660. Epub 2021, Feb 4. PMID: 33538338.
3. Herrera-Gayol A., Jothy S. Effects of hyaluronan on the invasive properties of human breast cancer cells in vitro. Int. J. Exp. Pathol. 2001 Jun. 82(3). 193-200. doi: 10.1046/j.1365-2613.2001.iep0082-0193-x. PMID: 11488992; PMCID: PMC2517708.
4. Hamilton S.R., Fard S.F., Paiwand F.F., Tolg C., Veiseh M., Wang C., McCarthy J.B., Bissell M.J., Koropatnick J., Turley E.A. The hyaluronan receptors CD44 and Rhamm (CD168) form complexes with ERK1,2 that sustain high basal motility in breast cancer cells. J. Biol. Chem. 2007, Jun 1. 282(22). 16667-16680. doi: 10.1074/jbc.M702078200. Epub 2007, Mar 28. PMID: 17392272; PMCID: PMC2949353.
5. Toole B.P. Hyaluronan-CD44 Interactions in Cancer: Paradoxes and Possibilities. Clin. Cancer Res. 2009, Dec 15. 15(24). 7462-7468. doi: 10.1158/1078-0432.CCR-09-0479. PMID: 20008845; PMCID: PMC2796593.
6. Al-Othman N., Alhendi A., Ihbaisha M., Barahmeh M., Alqaraleh M., Al-Momany B.Z. Role of CD44 in breast cancer. Breast Dis. 2020. 39(1). 1-13. doi: 10.3233/BD-190409. PMID: 31839599.
7. Carvalho A.M., Soares da Costa D., Paulo P.M.R., Reis R.L., Pashkuleva I. Co-localization and crosstalk between CD44 and RHAMM depend on hyaluronan presentation. Acta Biomater. 2021, Jan 1. 119. 114-124. doi: 10.1016/j.actbio.2020.10.024. Epub 2020, Oct 20. PMID: 33091625.
8. Misra S., Hascall V.C., Markwald R.R., Ghatak S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, May 6. 6. 201. doi: 10.3389/fimmu.2015.00201. PMID: 25999946; PMCID: PMC4422082.
9. Turley E.A. Hyaluronan and cell locomotion. Cancer Metastasis Rev. 1992 Mar. 11(1). 21-30. doi: 10.1007/BF00047600. PMID: 1380898.
10. Maxwell C.A., McCarthy J., Turley E. Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions? J. Cell. Sci. 2008, Apr 1. 121(Pt 7). 925-932. doi: 10.1242/jcs.022038. PMID: 18354082.
11. Tolg C., McCarthy J.B., Yazdani A., Turley E.A. Hyaluronan and RHAMM in wound repair and the “cancerization” of stromal tissues. Biomed. Res. Int. 2014. 2014. 103923. doi: 10.1155/2014/103923. Epub 2014, Aug 4. PMID: 25157350; –PMCID: PMC4137499.
12. Korkes F., de Castro M.G., de Cassio Zequi S., Nardi L., Del Giglio A., de Lima Pompeo A.C. Hyaluronan-mediated motility receptor (RHAMM) immunohistochemical expression and androgen deprivation in normal peritumoral, hyperplasic and neoplastic prostate tissue. BJU Int. 2014 May. 113(5). 822-829. doi: 10.1111/bju.12339. Epub 2013, Oct 31. PMID: 24053431.
13. Du W., Gerald D., Perruzzi C.A., Rodriguez-Waitkus P., Enayati L., Krishnan B., Edmonds J., Hochman M.L., Lev D.C., Phung T.L. Vascular tumors have increased p70 S6-kinase activation and are inhibited by topical rapamycin. Lab. Invest. 2013 Oct. 93(10). 1115-1127. doi: 10.1038/labinvest.2013.98. Epub 2013, Aug 12. PMID: 23938603.
14. Ge Y., Chen J. Mammalian target of rapamycin (mTOR) signaling network in skeletal myogenesis. J. Biol. Chem. 2012, Dec 21. 287(52). 43928-35. doi: 10.1074/jbc.R112.406942. Epub 2012, Oct 31. PMID: 23115234; PMCID: PMC3527976.
15. LoRusso P.M. Mammalian target of rapamycin as a rational therapeutic target for breast cancer treatment. Oncology. 2013. 84(1). 43-56. doi: 10.1159/000343063. Epub 2012, Oct 30. PMID: 23128843.
16. Li J., Kim S.G., Blenis J. Rapamycin: one drug, many effects. Cell. Metab. 2014, Mar 4. 19(3). 373-379. doi: 10.1016/j.cmet.2014.01.001. Epub 2014, Feb 6. PMID: 24508508; PMCID: PMC3972801.
17. Menon S., Manning B.D. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008 Dec. 27. Suppl. 2 (02). 43-51. doi: 10.1038/onc.2009.352. PMID: 19956179; PMCID: PMC3752670.
18. Ekizceli G., Uluer E.T., Inan S. Investigation of the effects of rapamycin on the mTOR pathway and apoptosis in metastatic and non-metastatic human breast cancer cell lines. Bratisl. Lek. Listy. 2020. 121(4). 308-315. doi: 10.4149/BLL_2020_049. PMID: 32356448.
19. Fırat F., Özgül M., Türköz Uluer E., Inan S. Effects of caffeic acid phenethyl ester (CAPE) on angiogenesis, apoptosis and oxidatıve stress ın various cancer cell lines. Biotech. Histochem. 2019 Oct. 94(7). 491-497. doi: 10.1080/10520295.2019.1589574. Epub 2019, Apr 17. PMID: 30991851.
20. Ozdemir A.T., Oztatlici M., Ozgul-Ozdemir R.B., Cakır B., Ozbilgin K., Dariverenli E., Kirmaz C. The effects of preconditioning with IFN-γ, IL-4, and IL-10 on costimulatory ligand expressions of mesenchymal stem cells. Int. J. Med. Biochem. 2021. 4(2). 121-130. doi: 10.14744/ijmb.2021.77487.
21. Hare S.H., Harvey A.J. mTOR function and therapeutic targeting in breast cancer. Am. J. Cancer Res. 2017, Mar 1. 7(3). 383-404. PMID: 28400999; PMCID: PMC5385631.
22. Sun S.Y., Rosenberg L.M., Wang X., Zhou Z., Yue P., Fu H., Khuri F.R. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005, Aug 15. 65(16). 7052-7058. doi: 10.1158/0008-5472.CAN-05-0917. PMID: 16103051.
23. Senbanjo L.T., Chellaiah M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell. Dev. Biol. 2017, Mar 7. 5. 18. doi: 10.3389/fcell.2017.00018. PMID: 28326306; PMCID: PMC5339222.
24. Afify A., Purnell P., Nguyen L. Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration, and invasion. Exp. Mol. Pathol. 2009 Apr. 86(2). 95-100. doi: 10.1016/j.yexmp.2008.12.003. Epub 2009, Jan 6. PMID: 19167378.
25. Bellerby R., Smith C., Kyme S., Gee J., Günthert U., Green A., Rakha E., Barrett-Lee P., Hiscox S. Overexpression of Specific CD44 Isoforms Is Associated with Aggressive Cell Features in Acquired Endocrine Resistance. Front. Oncol. 2016, Jun 20. 6. 145. doi: 10.3389/fonc.2016.00145. PMID: 27379207; PMCID: PMC4913094.
26. Chen C., Zhao S., Karnad A., Freeman J.W. The biology and role of CD44 in cancer progression: therapeutic implications. J. Hematol. Oncol. 2018, May 10. 11(1). 64. doi: 10.1186/s13045-018-0605-5. PMID: 29747682; PMCID: PMC5946470.
27. Bai J., Chen W.B., Zhang X.Y., Kang X.N., Jin L.J., Zhang H., Wang Z.Y. HIF-2α regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling. World J Stem Cells. 2020, Jan 26. 12(1). 87-99. doi: 10.4252/wjsc.v12.i1.87. PMID: 32110277; PMCID: PMC7031759.
28. Chen H., Mohan P., Jiang J., Nemirovsky O., He D., Fleisch M.C., Niederacher D., Pilarski L.M., Lim C.J., Maxwell C.A. Spatial regulation of Aurora A activity during mitotic spindle assembly requires RHAMM to correctly localize TPX2. Cell Cycle. 2014. 13(14). 2248-2261. doi: 10.4161/cc.29270. Epub 2014, May 29. PMID: 24875404; PMCID: PMC4111680.
29. Wang J., Li D., Shen W., Sun W., Gao R., Jiang P., Wang L., Liu Y., Chen Y., Zhou W., Wang R., Xiang R., Stupack D., Luo N. RHAMM inhibits cell migration via the AKT/GSK3β/Snail axis in luminal A subtype breast cancer. Anat. Rec. (Hoboken). 2020 Sep. 303(9). 2344-2356. doi: 10.1002/ar.24321. Epub 2019, Dec 10. PMID: 31769593.
30. Wang Z., Wu Y., Wang H., Zhang Y., Mei L., Fang X., Zhang X., Zhang F., Chen H., Liu Y., Jiang Y., Sun S., Zheng Y., Li N., Huang L. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc. Natl Acad. Sci. USA. 2014, Jan 7. 111(1). 89-98. doi: 10.1073/pnas.1319190110. Epub 2013, Dec 23. Erratum in: Proc. Natl Acad. Sci. USA. 2016, Nov 14. PMID: 24367099; PMCID: PMC3890879.
31. Schütze A., Vogeley C., Gorges T. et al. RHAMM splice variants confer radiosensitivity in human breast cancer cell lines. Oncotarget. 2016, Apr 19. 7(16). 21428-21440. doi: 10.18632/oncotarget.7258. PMID: 26870892; PMCID: PMC5008296.

/6.jpg)
/7.jpg)
/7_2.jpg)
/8.jpg)