Журнал «Почки» Том 10, №3, 2021
Вернуться к номеру
Підсумкові результати дослідження BIRCOV (БРА, ІАПФ, ПІР при COVID-19)
Авторы: D.D. Ivanov(1), M.D. Ivanova(1), T. Crestanello(2)
(1) — Shupyk National Healthcare University of Ukraine, Kyiv, Ukraine
(2) — Milan, Italy
Рубрики: Нефрология
Разделы: Клинические исследования
Версия для печати
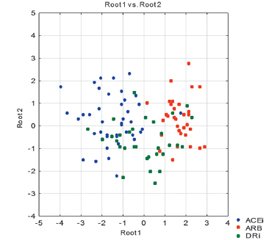
Актуальність. У літературі дискутується питання про можливий вплив інгібіторів ренін-ангіотензин-альдостеронової системи (іРААС) на стан людей з гіпертензією, які захворіли на COVID-19. Основою для такої дискусії є використання коронавірусом рецептора ангіотензинперетворюючого фермента 2 (АПФ) для проникнення в клітину. Три можливі механізми взаємодії іРААС з коронавірусом можуть бути реалізовані в клінічній практиці: погіршення перебігу інфекції, нейтральний або той, що допомагає організму чинити опір COVID-19. З огляду на різні механізми зниження тиску інгібіторами РААС можна очікувати й відмінностей в стані людей з COVID-19, які отримують названі препарати. Метою дослідження було вивчення клінічних особливостей і лабораторних показників у пацієнтів із гіпертензією 1–2-го ступеня, які отримували іРААС і захворіли на COVID-19. Матеріали та методи. Дослідження POEM (Докази, орієнтовані на пацієнта, що мають значення) проводилося як відкрите перспективне рандомізоване в двох медичних центрах у пацієнтів, які захворіли на COVID-19, що попередньо отримували іАПФ, або блокатори рецепторів ангіотензину (БРА), або прямі інгібітори реніну (ПІР) як основну антигіпертензивну терапію. Було обстежено 120 людей з гіпертонічною хворобою 1–2-ї стадії, 108 увійшли до дослідження BIRCOV. COVID-19 був підтверджений тестом ПЛР, спостереження за хворобою поділили на 2 періоди: до 12 тижнів і до 24 тижнів. Первинна кінцева точка: артеріальний тиск (АТ), який був відомий за тиждень до розвитку COVID-19 і контролювався під час початку захворювання, на 2, 4, 12, 24-й тижні після дебюту коронавірусної інфекції. Вторинні кінцеві точки — клінічні ознаки COVID-19. Окремо проведенй субаналіз у пацієнтів із хронічною хворобою нирок (ХХН). Результати. Усі пацієнти були рандомізовані в 3 групи й отримували: ІАПФ — 42 (39 %), БРА — 35 (32 %) або ПІР — 31 (29 %). Дослідження BIRCOV задокументувало тенденцію зниження АТ протягом перших двох тижнів захворювання на COVID-19 з поступовим поверненням до вихідних значень до 12-го тижня. 23 (21 %) пацієнти відмінили ліки на термін до 2 тижнів через тяжку гіпотензію. Однак показники АТ після COVID-19 у більшості учасників залишалися нижчими за вихідні протягом 4 тижнів. Застосування інгібіторів АПФ значно збільшувало ризик відміни порівняно з ПІР (RR 1,648; 95% ДІ 0,772–3,519; NNT 7,0) та БРА (RR 13,023; 95% ДI 1,815–93,426; NNT 2,9) при COVID-19. Синхронне зниження розрахункової швидкості клубочкової фільтрації (рШКФ) та систолічного АТ було більш вираженим у пацієнтів із ХХН. Найбільше зниження рШКФ було відзначене в людей, які приймали іАПФ. Зниження рШКФ коливалося від 23 % при ХХН стадії 1 до 45 % на 4-й стадії ХХН. Двом людям був потрібен короткочасний діаліз. Аналіз вторинних результатів показав, що в 23 % людей без попередньої альбумінурії дана патологія сформувалася в діапазоні А2. Протягом 12 тижнів спостереження у 81 % пацієнтів спостерігалася спонтанна ліквідація альбумінурії. Після COVID-19 (спостереження понад 12 тижнів) альбумінурія зберігалася в 19 % пацієнтів, 90 % з них мали ХХН. У пацієнтів із попередньо визначеною ХХН спостерігалося збільшення альбумінурії в 78 % випадків, і її повернення до вихідного рівня спостерігалося лише в 24 % пацієнтів до 12-го тижня та в 49 % через 24 тижні. Висновки. У людей із гіпертензією 1–2-го ступеня, що постійно отримують інгібітори РААС, при захворюванні на COVID-19 може розвинутись гіпотензія в разі прийому іАПФ. COVID-19 призводить до транзиторного виникнення альбумінурії та зниження швидкості клубочкової фільтрації, що особливо небезпечно для людей із ХХН.
Актуальность. В литературе дискутируется вопрос о возможном влиянии ингибиторов ренин-ангиотензин-альдостероновой системы (иРААС) на состояние людей с гипертензией, которые заболели COVID-19. Основой для такой дискуссии является использование коронавирусом рецептора ангиотензинпревращающего фермента 2 (АПФ) для проникновения в клетку. Три возможных механизма взаимодействия иРААС с коронавирусом могут быть реализованы в клинической практике: усугубляющий течение инфекции, нейтральный или помогающий организму. Учитывая различный механизм снижения давления ингибиторами РААС, можно ожидать и различия в состоянии людей с COVID-19, получающих названные препараты. Целью исследования явилось изучение клинических особенностей и лабораторных показателей у пациентов с гипертензией 1–2-й степени, получавших иРААС и заболевших COVID-19. Материалы и методы. Исследование POEM (Доказательства, ориентированные на пациента и имеющие значение) проводилось как открытое проспективное рандомизированное исследование в двух медицинских центрах с участием людей, которые заболели COVID-19 и получали иАПФ, блокаторы рецепторов ангиотензина (БРА) или прямые ингибиторы ренина (ПИР) в качестве базовой антигипертензивной терапии. Отобрано 120 человек с гипертензией 1–2-й стадии, 108 из них участвовали в исследовании BIRCOV. COVID-19 был подтвержден с помощью ПЦР-теста, наблюдение за заболеванием разделено на 2 периода: до 12 недель и до 24 недель. Первичная конечная точка: артериальное давление (АД), которое было известно за неделю до COVID-19 и затем мониторировалось во время начала заболевания, на 2, 4, 12, 24-й неделе от его дебюта. Вторичными конечными точками были клинические характеристики. Отдельно был проведен субанализ пациентов с хронической болезнью почек (ХБП). Результаты. Все пациенты были рандомизированы в 3 группы, которые соответственно получали: ИАПФ — 42 (39 %), БРА — 35 (32 %) или ПИР — 31 (29 %). Исследование BIRCOV зафиксировало тенденцию к снижению АД в первые две недели заболевания COVID-19 с его постепенным возвращением к исходным значениям вплоть до 12-й недели. У 23 (21 %) пациентов был отменен прием лекарств на срок до 2 недель из-за тяжелой гипотензии. Однако значения АД после COVID-19 у большинства испытуемых оставались ниже исходного уровня в течение 4 недель. Использование ингибиторов АПФ значительно увеличивало риск синдрома отмены по сравнению с ПИР (ОР 1,648; 95% ДИ 0,772–3,519; NNT 7,0) и БРА (ОР 13,023; 95% ДИ 1,815–93,426; NNT 2,9) из-за COVID-19. Синхронное снижение расчетной скорости клубочковой фильтрации (рСКФ) и систолического АД было более выражено у пациентов с ХБП. Наибольшее снижение рСКФ было отмечено у людей, принимавших иАПФ. Снижение рСКФ варьировало от 23 % при ХБП 1-й стадии до 45 % при ХБП 4-й стадии. Два человека нуждались в кратковременном диализе. Анализ вторичной конечной точки показал, что у 23 % людей без предшествующей альбуминурии она появилась в диапазоне А2. В течение 12 недель наблюдения у 81 % пациентов наблюдалась спонтанная ликвидация альбуминурии. После COVID-19 (сроки наблюдения свыше 12 недель) альбуминурия сохранялась у 19 % пациентов, 90 % из них имели ХБП. У пациентов с предшествующей ХБП наблюдалось увеличение альбуминурии в 78 % случаев, а ее возврат к исходному уровню отмечен только у 24 % пациентов к 12-й неделе и 49 % через 24 недели. Выводы. У людей с гипертензией 1–2-й степени, постоянно получающих ингибиторы РААС при заболевании COVID-19, может развиваться гипотензия при приеме иАПФ. COVID-19 приводит к транзиторному возникновению альбуминурии и снижению скорости клубочковой фильтрации, что особенно опасно для людей с ХБП.
Background. The question of the possible effect of the inhibitors of the renin-angiotensin system (iRAS) on hypertensive subjects who fell ill with COVID-19 has been discussed in the literature. SARS-CoV-2 is well-known to use an angiotensin-converting enzyme 2 receptors facilitating virus entry into host cells. There are three possible mechanisms of angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) effect in COVID-19 in clinical practice: with worsening, neutral, or helpful function. Considering the different mechanisms of blood pressure reduction by iRAS, one can expect differences in people with COVID-19 receiving these drugs. The purpose of the BIRCOV study is to pinpoint possible clinical and laboratory differences in hypertensive people who received iRAS and suffered from coronavirus infection. Materials and methods. Patient-Oriented Evidence that Matters (POEM) intervention was designed as an open prospective randomized two medical centers trial in subjects suffering from COVID-19 who have been receiving iRAS, either ACEi, ARB, or direct renin inhibitor (DRi) as basic antihypertensive therapy. One hundred and twenty people with stage 1–2 hypertension have been screened, 108 subjects were enrolled in the BIRCOV study. COVID-19 was confirmed by a PCR test; the disease follow-up was divided into 2 periods: up to 12 weeks and up to 24 weeks. The primary outcome measure was as follows: blood pressure (BP) was known one week before COVID-19 onset and was measured during the disease on weeks 2, 4, 12, 24. The secondary outcome measures were clinical features. Subanalysis in patients with chronic kidney disease (CKD) was performed. Results. All patients were randomized into 3 groups who received: ACEi — 42 (39 %), ARB — 35 (32 %), or DRi — 31 (29 %). The BIRCOV trial documented the trend of BP lowering in the first two weeks of the COVID-19 disease with its gradual return to baseline values up to the 12th week. Twenty-three (21 %) patients have withdrawn medicine for up to 2 weeks due to severe hypotension. However, the BP values after COVID-19 in most subjects remained lower than the baseline ones for 4 weeks. The use of ACE inhibitors significantly increased the risk of withdrawal compared to DRi (RR 1.648; 95% CI 0.772–3.519; NNT 7.0) and ARB (RR 13.023; 95% CI 1.815–93.426; NNT 2.9) due to COVID-19. The synchronous decline of estimated glomerular filtration rate (eGFR) and systolic BP was more pronounced in CKD patients. The greatest decrease in eGFR was noted in people who have been taking ACEi. The drop in eGFR ranged from 23 % in CKD stage 1 to 45 % in CKD stage 4. Two people required short-term dialysis. The analysis of secondary outcome points demonstrated that in 23 % of people without preceding albuminuria it developed in the A2 range. During 12 weeks of observation, 81 % of patients had spontaneous albuminuria reduction. Post-COVID-19 (above 12 weeks) albuminuria remained in 19 % of patients, 90 % of them had a history of CKD. Patients with preceding CKD had an increase in albuminuria in 78 % of cases, and its return to the baseline was observed only in 24 % of patients by the 12th week and in 49 % of individuals in 24 weeks. Conclusions. People with stage 1–2 hypertension who are receiving chronic iRAS and suffer from COVID-19 may develop hypotension with ACE inhibitors. COVID-19 leads to transient albuminuria and decreased glomerular filtration rate, which is especially dangerous for people with CKD.
інгібітори ренін-ангіотензин-альдостеронової системи; інгібітори ангіотензинперетворюючого фермента; блокатори рецепторів ангіотензину; прямі інгібітори реніну; COVID-19; дослідження BIRCOV
ингибиторы ренин-ангиотензин-альдостероновой системы; ингибитор ангиотензинпревращающего фермента; блокаторы рецепторов ангиотензина; прямые ингибиторы ренина; COVID-19; исследование BIRCOV
renin-angiotensin system inhibitors; angiotensin receptor blockers; angiotensin-converting enzyme inhibitors; direct renin inhibitor; COVID-19; BIRCOV trial
Background
Materials and methods
/40.jpg)
Results
Clinical features arm
Discussion
Conclusions
- www.era-edta.org/en/covid-19-news-and-information/#toggle-id-8
- Reynolds H.R., Adhikari S., Pulgarin C.S., Troxel A.B., Iturrate E., Johnson S.B., Anaïs Hausvater, Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I. et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. The NEJM. May 1, 2020. doi: 10.1056/NEJMoa2008975.
- Lei Y., Zhang J., Schiavon C.R., He M., Chen L., Shen H., Zhang Y., Yin Q. et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circulation Research. 2021. 128. 1323-1326. doi: 10.1161/CIRCRESAHA.121.318902.
- Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., Cinatl J., Münch C. SARS-CoV-2 infected host cell proteomics reveal potential therapy targets. Nature. 2020. doi: 10.21203/rs.3.rs-17218/v1.
- Davidson A.D., Williamson M.K., Lewis S. et al. Characterisation of the transcriptome and proteome of SARS-CoV-2 reveals a cell passage induced in-frame deletion of the furin-like cleavage site from the spike glycoprotein. Genome Med. 2020. 12, 68. doi: 10.1186/s13073-020-00763-0.
- http://www.nephjc.com/news/covidace2
- Mehra M.R., Desai S.S., SreyRam Kuy, Henry T.D., Patel A.N. Сardiovascular Disease, Drug Therapy, and Mortality in Covid-19 The NEJM. 2020. May 1. doi: 10.1056/NEJMoa2007621.
- Mourad J., Levy B.I. Interaction between RAS inhibitors and ACE2 in the context of COVID-19. Nat. Rev. Cardiol. 2020. doi: 10.1038/s41569-020-0368-x.
- https://wilkes.libguides.com/c.php?g=191942&p=1266516
- Ivanova M.D., Gozhenko A.I., Crestanello T., Ivanov D.D. Early Coaching to Increase Water Intake in CKD. Annals of nutrition & metabolism. 2020. 76. 69-70. doi: 10.1159/000515276.
- https://www.gigacalculator.com/calculators/
- https://qxmd.com/calculate/calculator_308/kidney-failure-risk-equation-4-variable
- http://www.nephjc.com/covid19
- www.cebm.net/covid-19/angiotensin-converting-enzyme-ace-inhibitors-and-angiotensin-receptor-blockers-in-covid-19
- Cohen J.B., South A.M., Shaltout H.A., Sinclair M.R., Sparks M.A. Renin-angiotensin system blockade in the COVID-19 pandemic. Clinical Kidney Journal. 2021 March. Vol. 14, Issue Supplement 1. i48-i59. doi: 10.1093/ckj/sfab026.
- Najmeddin F., Solhjoo M., Ashraf H. et al. Effects of Renin-Angiotensin-Aldosterone Inhibitors on Early Outcomes of Hypertensive COVID-19 Patients: A Randomized Triple-Blind Clinical Trial. Am. J. Hypertens. 2021 Jul 15. hpab111. doi: 10.1093/ajh/hpab111.
- ERA-EDTA Council, ERACODA Working Group, Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrology Dialysis Transplantation. 2021 January. Vol. 36, Issue 1. 87-94. doi: 10.1093/ndt/gfaa314.
- Chung E.Y., Palmer S.C., Natale P. et al. Incidence and Outcomes of COVID-19 in People With CKD: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2021 Aug 5. S0272-6386(21)00771-X. doi: 10.1053/j.ajkd.2021.07.003.
- Jia N., Zhang G., Sun X. et al. Influence of angiotensin converting enzyme inhibitors/angiotensin receptor blockers on the risk of all-cause mortality and other clinical outcomes in patients with confirmed COVID-19: A systemic review and meta-analysis. J. Clin. Hypertens. (Greenwich). 2021 Jul 28. doi: 10.1111/jch.14329.
- Lopez-Leon S., Wegman-Ostrosky T., Perelman C. et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci. Rep. 2021 Aug 9. 11 (1). 16144. doi: 10.1038/s41598-021-95565-8.

/39.jpg)
/39_2.jpg)
/40_2.jpg)
/41.jpg)