Introduction
Viral hepaitites are still one of the major health problems around the world today. It is estimated that about 257 million people in the world live with chronic hepatitis B (HBV) infection, and in recent years the death rate due to Hepatitis B complications reaches 900,000 people each year [1].
Hepatitis B is a life-threatening liver infection. It can cause chronic infections. It increases the risk of death caused by cirrhosis and hepatocellular carcinoma (HCC). The most effective way to control hepatitis B infection is to prevent transmission and ensure immunity [2–4]. A safe and effective vaccine is available that provides 98–100 % protection against hepatitis B. Prevention of hepatitis B infection prevents the development of complications, including chronic disease and liver cancer. Routine infant immunity against hepatitis B (3 doses) has reached 84 % global coverage as of 2017 according to World Health Organization recommendations [1]. In our country, the Hepatitis B vaccine was included in the routine vaccination program by the Ministry of Health in 1998 [5].
Turkey is considered a middle endemic country according to HBV prevalence classification [6].
In our country, Hepatitis positivity in pregnant women between 1987–2004 was reported between 3.5 to 9.3 % [6]. In more recent studies, it is observed that the positivity of hepatitis in pregnant women is between 1.9 to 9.4 % (average 4.3 %) [7]. As HBV parenteral (percutaneous contact with infected blood or body fluids), horizontally (intimate contact without intercourse), sexual and perinatal or vertically (from mother to child) the virus can pass. In high endemic areas, perinatal transmission is one of the most important pathways of HBV spread. Intrauterine infection of the fetus is unlikely due to the placental barrier. HBV transmission during or after birth, usually in epithelial and mucosal surfaces, infected maternal fluids, contact with the maternal blood swallowed while passing through the vaginal canal, through the placenta during cesarean delivery, or a damaged contact occurs in contact with the mother’s blood [8]. When the mother is infected with acute disease, the risk of infection for the baby reaches 10 % in the first 2 trimesters, 80–90 % in the last trimester and prenatal period [9]. About 50 % of pregnant women with chronic HBV infection infect their babies during the perinatal period. Maternal HBV positivity and HBV DNA levels are important in perinatal transmission. The risk of HBV transmission from an HBV-positive mother is 70–90 %, while for an HBV-negative mother, this figure is about 10–40 %. As the Viral load increases, the risk of HBV transmission also increases [10, 11]. Although breast milk contains HBV, it does not pose an additional risk for Hepatitis B infection of the child [12].
Perinatal transmission can be prevented by 95 % by early active and passive immunprofilaxis of the baby (Hepatitis B vaccination and hepatitis B hyperimmoglobulin (HBIG) administration). It is important to look at the serology of Hepatitis B in all pregnant women before birth [13–17].
The purpose of this study is to check whether the children are protected against Hepatitis B infection in the follow-up of Hepatitis B Immunoglobulin (HBIG) and Hepatitis B vaccine administered to babies born from HbsAg positive mothers who gave birth between January 2013 and December 2018 in our hospital, and to check the time of postnatal Hepatitis B immunoglobulin administration. To evaluate whether the baby is given a bath in the delivery room, breastfeeding, and whether it enhances the effectiveness of hepatitis B immunoglobulin and hepatitis B vaccine in protection against hepatitis B infection. In addition, it was aimed to evaluate the physical development of babies by looking at height and weight and to evaluate the relationship of these results with parameters such as maternal age, gestational week, birth type and weight.
Materials and methods
This study was conducted by Istanbul Health Sciences University Dr. It is a study conducted on babies born to mothers with chronic Hepatitis B between 01/01/2013–01/31/2018 at Sadi Konuk Training and Research Hospital. Postnatal vaccine and immunoglobulin administration status and time of administration, antibody response of infants to Hepatitis B vaccine, antibody formation or risk factors affecting these conditions in case of chronic Hepatitis B were evaluated retrospectively. It was planned as an intervention study to ensure the vaccination of those who did not respond to antibodies and the follow-up of the caught chronic Hepatitis B cases in the pediatric gastroenterology outpatient clinic.
This study was conducted by Istanbul Health Sciences University Dr. It was performed in Sadi Konuk Training and Research Hospital delivery room and pediatric gastroenterology outpatient clinic. In socioeconomic terms, our hospital serves all patients, especially the low-medium socioeconomic group. The hospital has a Baby Friendly Hospital certificate. Mothers are involved in childbirth, baby care, breast milk, breastfeeding training, etc. It is trained in many subjects.
For the work permit, Istanbul Health Sciences University Dr. An application was made to the Clinical Research Ethics Committee of Sadi Konuk Training and Research Hospital. Permission was obtained with the decision of the board of directors dated 20.05.2019.
55 cases born to a mother diagnosed with chronic Hepatitis B followed in the pediatric gastroenterology outpatient clinic of Istanbul Health Sciences University Dr. Sadi Konuk Training and Research Hospital between 01/01/2013–31/12/2018 were included in our study.
Anti-HCV, anti-HIV, AST and ALT levels were also evaluated in 55 patients participating in the study, but these tests were not considered as criteria for participation in the study.
For this study, information about mothers and babies diagnosed with chronic hepatitis B was scanned from the computer registry system of the hospital. From the records,
— The mother’s gestational age.
— Maternal Hepatitis B test.
— Additional maternal viral disease studies (Hepatitis C, HIV).
Delivery method:
— Baby birth weight.
— Baby birth week.
Hepatitis B vaccine and immunoglobulin administration time.
Bathroom situation in the delivery room.
Infant Hepatitis B tests:
— Additional studies on viral diseases of the child (Hepatitis C, HIV).
— Infant’s liver transaminase tests (AST, ALT).
Information has been reached.
To the families of the cases:
— Baby’s immunization schedule.
— Checking the mother’s hepatitis B status.
— The time of the baby’s breast milk intake.
— Baby’s height and weight.
— Follow-up and medication use status of the mother during pregnancy was asked.
Babies born to mothers with chronic hepatitis B infection were called to the hospital for examination. The babies of the families who agreed to participate in the study were examined.
In the study, those with HBsAg test results > 1.0 were considered positive, and those < 0.9 were considered negative. Anti-HBS test result was evaluated as antibody titer > 10 IU/ml positive and < 10 IU/ml negative. Anti-HCV value was evaluated as > 1.0 positive and < 0.9 negative. Normal value ranges for AST and ALT were taken as 0–40 U/L (units/liter).
Children who did not agree to participate in the study and children previously diagnosed with chronic B hepatitis were excluded from the study.
Statistical analysis
“Statistical Package for Social Sciences — SPSS 25” software was used to evaluate the results. The Student t test was used to calculate the mean, standard deviation, median, frequency and ratio from descriptive statistical methods, as well as to compare parametric variables while evaluating the study data. Comparison of categorical variables was made with chi-square test and Fisher exact test. Statistical significance was considered p < 0.05
Results
A total of 11554 live births took place in our hospital between 01/01/2013–01/31/2018. According to the records, 94 mothers with chronic hepatitis B infection gave birth between these dates.
Figure 1 shows the number of live births by years, the number of mothers with chronic hepatitis B, and the numbers and percentages of people participating in the study.
The mean gestational age of the mothers participating in the study was 29.9 ± 6.0, the mean week of gestation was 37.7 ± 1.7, the mean birth weight of the babies was 3163.0 ± 558.1 g, the median number of pregnancies in mothers was 3 (min: 1, max: 7).
Table 1 shows the age of the mothers, the gender of the babies, the gestational week of the mother, the weight and shape of the babies, the hours of IG administration to the babies, the bath conditions in the delivery room and the breastfeeding times of the babies.
40 % (n = 22) of the babies were girls and 60 % (n = 33) were boys.
3.6 % of the babies had a birth weight below 2000 grams (n = 2) and 96.4 % had a birth weight above 2000 grams (n = 53).
56.4 % (n = 31) of the deliveries were realized by normal spontaneous vaginal delivery (nsvd) and 43.6 % (n = 24) by cesarean (C/S).
Hepatitis B vaccine and hyperimmunglobulin were administered to all of these babies. Hepatitis B vaccine was administered to all babies immediately after birth with hepatitis B hyperimmunglobulin 83.7 % (n = 46) in the first 12 hours after birth and 16.3 % (n = 9) after the 12th hour. (fig. 2).
While 41.8 % (n = 23) of the babies had a bath in the delivery room, 58.2 % (n = 32) did not take a bath.
According to family information, 12.7 % (n = 7) of these babies received breast milk in less than 6 months, and 87.3 % (n = 48) received breast milk for 6 months or more.
Hepatitis B vaccination schedule was given to 2 babies born below 2000 G in total of 4 doses at 0, 0, 1, 6 months, and 53 babies born above 2000 G, 3 doses at 0, 1, 6 months.
When the results are examined, out of 55 babies born to mothers with chronic hepatitis B infection, 8 (14.5 %) were between 0–12 months, 19 (34.5 %) were between 12–24 months, 3 (5 %, 4) 24–36 months, 7 (12.8 %) were between 36–48 months and 18 (32.8 %) were older than 48 months (fig. 2).
Hepatitis b infection was positive in 2 (3.6 %) of 55 babies included in the study. In 53 (96.4 %) cases with negative HBsAg, the titer level ranged from 0.42 to 0.83, with a mean of 0.55 ± 0.08 and a median of 0.54. In two HBsAg positive cases, the titer levels were 83.67 and 86.20 with a mean of 84.93 ± 1.78.
It was found that the mothers of both babies who were HBsAg positive had > 100 000 000 copies of positive HBV DNA during pregnancy.
While 43 (78.2 %) of the cases had a protective anti-HBs antibody titer (10 UI/mL), 12 (21.8 %) had no protective antibody titer (< 10 UI/mL) (fig. 2).
The level of anti-HBs titer observed ranged from 0 to 795 IU/mL, with a mean of 94.3 ± 144.9 IU/mL and a median of 38.6 IU/mL.
Of the 12 babies without protective antibody titres, four were premature and eight were term babies.
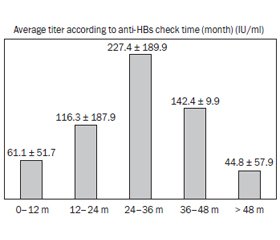
When the anti-HBs titres were examined according to the examination period, the mean anti-HBs was 61.1 ± 51.7 IU/mL in infants aged 0–12 months, 116.3 ± 187.9 IU/mL in those examined between 12–24 months, and 227.4 in children aged 24–36 months. It was found as ± 198.9 IU/mL, 142.4 ± 9.9 IU/mL in those examined between 36–48 months and 44.8 ± 57.9 IU/mL in those older than 48 months (fig. 3).
Anti-HBs titer levels were compared according to the characteristics of the mothers and babies (table 2).
There was no statistically significant relationship between the mothers' ages and anti-HBs titer levels (p > 0.05).
There was no statistically significant difference between anti-HBs titre levels according to the week of gestation (p > 0.05).
No statistically significant difference was found between NSD and C/s in anti-HBs titer levels according to the delivery method (p > 0.05).
There was no statistically significant difference between anti-HBs titer levels according to the gender of the baby (p > 0.05).
There was no statistically significant difference between the anti-HBs titer levels of babies who were admitted for the first 12 hours according to the Hepatitis B Hyperimmunglobulin hours and after (p > 0.05).
There was no significant difference in anti-HBs titer levels between those who were washed and those who were not washed in the delivery room (p > 0.05).
There was no significant difference between breast milk and anti-HBs titer levels according to the feeding time (p > 0.05).
The comparison could not be made because the number of babies with a birth weight below 2000 G was insufficient.
While 45 (81.8 %) of 55 mothers diagnosed with chronic hepatitis B were followed up by an Infection and Clinical Microbiology or Gastroenterology specialist, 38 (69.1 %) used medication during pregnancy. It was found that 89.5 % (n = 34) of the mothers who took the drug used Tenofovir and 10.5 % (n = 4) used lamivudine (table 3).
There is a statistically significant relationship between the status of mother follow-up and the status of baby follow-up (p < 0.001). Likewise, there is a statistically significant relationship between the use of medication by mothers and follow-up status of babies (p < 0.001). It was observed that mothers who were self-monitored and used medication followed their babies significantly higher than mothers who did not (table 3).
Discussion
Chronic hepatitis B is one of the most common and important health problems in the world. According to the WHO, 257 million people are thought to live with chronic hepatitis B infection each year, and about 900 000 die each year due to complications related to Hepatitis B. It is in the middle endemicity class. The risk of chronic, therefore, complications, increases as the age of infection decreases [18, 19]. In case of infection, chronic disease is observed in the neonatal period at 80–90 %, in young ages (1–4 years) at 30 %, and in adults at 5 %. For this reason, preventing the virus at an early age is one of the most important steps in protection from Hepatitis B. From this point of view, it is of great importance that the Hepatitis B vaccine is routinely administered to all newborn children. In addition, pregnant women should be screened for HBV, carrier and sick mothers should be detected, and their babies should be given hepatitis B vaccine and HBIG at birth [20]. Immunization protects these babies from HBV infection by 85–95 %. After vaccination, babies should be evaluated with hepatitis and anti-HBs tests when they are 9–15 months old and vaccinated again if necessary [21].
Looking at various studies conducted in the world and in Turkey, it was observed that Seroprevelane of Hepatitis B varies depending on the region and years in which the study was conducted. Kaya et al. study of 12 092 blood donors in Trabzon province between 2004 and 2007, he showed Hepatitis B seroprevalence as 1.6 % [22]. Araz et al. in their study with 11 840 pregnant women in Gaziantep between 2003 and 2005, they showed Hepatitis B seroprevelan as 2.1 % [23]. Köksaldı et al. in a study conducted in Hatay province in 2009, they showed Hepatitis B seroprevalence in 5410 pregnant women as 1.5 % [24]. Köse et al. in their study with 2003 pregnant women in Izmir between 2010 and 2011, they showed Hepatitis B seroprevalence as 1.14 % [25]. Furuncuoglu et al. They divided 7605 pregnant women who gave birth between 1995–2005 into 3 study periods as those who gave birth between 1995–2001, 2002–2008 and 2009–2015, and showed that seroprevalence decreased from 2.6 to 0.8 % between the first and last study period [26]. In a study published in 2005 from Greece, Ioannis et al. they screened for HBV in 13581 women during fertility and found the rate of Hbags to be 1,156 % [27]. Jensen et al. in their study in Denmark, located in the region of low endemicity, Hepatitis was positive in 0.43 % of 4098 pregnant women [28]. In our study, perinatally transmitted Hepatitis B seroprevalence was found to be 0.8 %. Although this rate was high compared to studies conducted in the region of low endemicity, it was found to be low compared to studies conducted in Turkey. This widespread and routine immunization of hepatitis B vaccine will be included in the program, also may be related to the increase of social awareness.
All babies taken in the study were immunized according to the Ministry of Health’s Expanded Immunization Program (Hepatitis B vaccine at birth and HBIG were made intramuscularly from different limbs, and then 0, 1, 6 for babies born 2000 grams and above. Nov. month, 0.0, 1, 6 for babies with birth weight less than 2000 grams. They had been vaccinated in accordance with the lunar vaccination scheme).
In the world, there are studies that compare the protection of Hepatitis B vaccine and Hepatitis B vaccine + HBIG to infants born to hepatitis-positive mothers, especially in areas of high endemicity. Yang et al. in their study in Taiwan, they compared only the groups that were vaccinated and the vaccine + HBIG and postnatal 7. compared to the anti-HBs titer averages looked at per month, they showed that only the vaccinated group had higher titer averages [29]. This suggests that HBIG may suppress the active vaccine response. In our country, in accordance with the circular of the Ministry of Health, Hepatitis B vaccine and HBIG are routinely administered at birth to infants of hepatitis-positive mothers. Both hepatitis B vaccine and HBIG because all of us have our babies we haven't done such a comparison applies.
In Perinatal transmission, the titer level of HBV in the mother, the level of HBV, the number of copies of HBV DNA in the mother’s serum, and the positivity of HBV in the cord blood are effective. HBeAg can pass through the placenta, but in most babies, this HBeAg clears and becomes negative within 6 months. If the mother’s viral load is too high, the child can become a chronic carrier even if the vaccine and immunoglobulin are administered. Zhang et al. in a study conducted by 41 hepatitis positive pregnant women in China, 14 of these pregnant women were found to be positive for HCV, and 27 pregnant women were found to be negative for HBV. When cord blood was examined, 6 babies born to an HBsAg-positive mother (42.9 %) were found to be positive for HBsAg, 1 baby born to an Hbags-negative mother (3.7 %) was found to be positive for HBsAg. As a result of this study, it was thought that babies born to an HBsAg-positive mother were more likely to be exposed to intrauterine Hepatitis B. Wiseman et al. in a study conducted by 313 hepatitis-positive pregnant women in Australia, transmission occurred in 4 babies, and it was shown that the mothers of all of these babies were positive for HBV DNA > 100 000 000 copies and HBV during pregnancy [30]. In our study, transmission was observed in 2 babies and the Mothers of these babies had positive HBV DNA > 100 000 000 copies during pregnancy.
In a study conducted by Qiou et al. With the children of 4112 HbsAg positive mothers in China, mothers were grouped as 18–24, 25–34, 45–45 years according to their gestational age, and when the anti-HBs levels of the children were compared with the mother age groups, there was not a statistically significant difference between the groups detected [31]. Similarly, in our study, mothers were divided into two groups under the age of 30 and under the age of 30 and above. No statistically significant difference was found between the levels of anti-HBs in children of the two groups (p = 0.803).
A study conducted by Stephan C. Ko and colleagues in the United States examined 8654 babies born to hepatitis-positive mothers and divided into two groups: those who were under 37 weeks of gestation week and those who were 37 weeks and above. Statistically, there was no significant difference between anti-HBs levels when evaluating vaccine responses [32]. Belloni et al. Conducted a study by vaccinating 2009 babies born to HBsAg (–) mothers with a total of 3 doses of recombinant Hepatitis B vaccine on day 4, when they were 1 and 6 months old, and these babies were under 38 weeks, 38 weeks and over, 2500 grams They divided it into groups of 2500 grams and above. When comparing anti-HBs levels in these babies, they did not find a statistically significant difference between birth weight and week of birth and anti-HBS titer levels [33]. In our study, cases were divided into two groups of 37 weeks six and 37 weeks and above according to gestation weeks. Vaccine responses of these children were compared with anti-HBs titers, and no statistically significant difference was found in support of previous studies (p = 0.946). But the comparison could not be made because the number of low birth weight babies was not sufficient.
There are studies investigating whether there is a difference in perinatal transmission between NSVD and C/S births. Yang et al.in a study conducted in China, 5105 of 9906 mothers with Hepatitis B gave birth with C/s and the remaining 4801 with NSVD. In the study of the serologies of infants born from these mothers in terms of Hepatitis B, the perinatal HBV transition rate was found to be 4.37 % in the group born with C/S and 9.31 % in the group born with NSVD. Accordingly, it has been shown that C/S birth reduces perinatal transmission at a statistically significant level compared to NSVD birth [34]. Similarly, in the meta-analysis in which Chang et al. Included 10 studies, it was observed that perinatal HBV transmission was 38 % less than C / S delivery with NSVD [35]. In our study, both of our two babies with perinatal transmission were born with NSVD. This also supports previous studies. Perinatal transmission is mostly caused by microperfusion of blood into the fetal circulation as a result of uterine contractions and rupture of the placenta during childbirth. Other possible causes may be swallowing amniotic fluid, vaginal secretion during vaginal delivery, and exposure to maternal blood. Since placental contraction and feto-maternal blood transfusion will be less at birth with elective C/s, it can be considered that the risk of transmission is less. Compared to anti-HBS titers, there was no statistically significant difference between NSVD and C/S babies (p = 0.590).
Although current guidelines recommend that HBIG be performed in the first 12 hours, the time of implementation and the number of studies in which Protection is evaluated are limited. Beasley et al.classified babies born from a HBIG-positive mother as those made before and after 48 hours of HBIG administration and showed that the perinatal transmission rate of those made before 48 hours was significantly lower than those made after 48 hours, while the anti-HBs titer average was significantly higher [36]. All of the babies involved in our study had HBIG administered before 24 hours. HBIG was applied to one of the two babies with perinatal transmission at postnatal 3rd hour and the other at postnatal 16th hour. Therefore, the comparison could not be made. Compared to anti-HBS titers, no statistically significant difference was found between HBIG levels before 12 hours and titre levels after 12 hours (p = 0.995). Although it is impossible to make a definitive comment with the limited data we have, implementing HBIG as soon as possible can be considered an appropriate approach.
Breast milk stimulates the immune system through antibody and lymphocyte transfer and increases vaccine response. But breast milk can also carry HBsAg. For this reason, there are studies that assess whether breast milk poses additional risk in maternal infants with HBV and its effect on vaccine response. Hill et al.’s study of 369 babies born from a mother with chronic hepatitis B found that 101 of the babies were fed with breast milk, while the other 268 babies were fed with formula. The HBV serologies of these babies were looked at. As a result, Hepatitis was not observed in any of the breast-fed babies, while Hepatitis was positive in 9 babies fed with formula. In this way, it has been shown that breastfeeding by their mothers of babies undergoing appropriate HBV Prevention does not pose an additional risk of HBV transmission [37]. The American Academy of Pediatrics also recommends that the baby can be fed with breast milk after the HBIG and Hepatitis B vaccine is administered as soon as the baby is born [38]. Chen et al. in a study of 546 babies born from an HBV-infected mother, 397 babies were fed with breast milk and the remaining 149 babies were fed with formula. Anti-HBs titers of these babies were found to be statistically significantly higher than the average anti-HBs titer of breast-fed babies compared to those fed with formula food [39]. In a study conducted at Goztepe educational and Research Hospital in 2009, 10 of the 50 babies born to an HbsAg-positive mother were with fed breast milk for less than 6 months, and the remaining 40 were fed with breast milk for 6 months and over. The only one of these babies who was positive for Hepatitis has never received breast milk. No statistically significant difference between the two groups was observed when anti-HBS titers were compared [40]. In our study, babies were divided into two groups: those under 6 months and those over 6 months according to their breast milk intake status. No significant difference was found between the two groups compared to anti-HBs titer levels (p = 0.323). Similarly, two babies who were positive for HbsAg were fed with breast milk less than 6 months old.
In addition, in our study, the median duration of breast milk intake was 2.1 months and the average total breast milk intake was 14.8 ± 8.9 months and the median was 13 months. Compared to TNSA 2018 data, no statistically significant difference was found with Turkey data in terms of breast milk intake times. This suggests that mothers do not have any drawback about feeding their babies with breast milk and continue to give breast milk safely. This gladsome situation can be attributed to the effectiveness of breastfeeding policies, baby-friendly hospital practices, continuous mother education and 1st step baby monitoring programs.
Another condition that is as important as immunprofilaxis of babies born to a mother infected with HBV is outpatient follow-up of these babies. Le STT et al. in his study, 451 mothers diagnosed with chronic hepatitis B and 454 babies born from these mothers were examined and it was shown that mothers who had their own follow-up regularly had their babies followed more regularly [41]. In our study, mothers who had their own follow-up and/or drug use were significantly higher than those who did not follow-up their babies (p < 0.001).
Chen et al. in their study with 147 hepatitis positive pregnant women, they showed that rapid cleansing of the baby's skin immediately after birth reduced the risk of perinatal transmission [42]. In our study, no significant association was found between Anti-HBs titre levels between babies who were bathed in the delivery room and babies who were not (p = 0.356). Both babies, who were positive for HbsAg, were bathed in the delivery room.
Conclussion
Inclusion of hepatitis B vaccine in the routine vaccination program, increasing public awareness and regular follow-up of people with HBV infection will reduce the prevalence of Hepatitis B infection. Mothers with chronic HBV infection should be closely monitored and if deemed necessary, drug therapy should be initiated to reduce viral load. These mothers should be informed in detail about the follow-up of their babies.
It is of great importance to look at serological tests in babies born to mothers with HBV infection in the 9th month after birth. Although studies on the prevention of HBV perinatal transmission of HBIG are limited, it is recommended that it be administered as soon as possible. Mothers should continue to give breast milk safely.
Received 20.03.2021
Revised 31.03.2021
Accepted 09.04.2021
/12.jpg)

/10.jpg)
/11.jpg)
/11_2.jpg)
/12_2.jpg)
/13.jpg)