Журнал «Здоровье ребенка» Том 14, №8, 2019
Вернуться к номеру
Патогенетична роль нітрозативного і оксидативного стресу в розвитку анемії запалення в дітей раннього віку
Авторы: H.O. Lezhenko, A.V. Abramov, A.O. Pohribna
Zaporizhzhia State Medical University, Zaporizhzhia, Ukraine
Рубрики: Педиатрия/Неонатология
Разделы: Клинические исследования
Версия для печати
Актуальність. Мета: вивчення патогенетичної ролі нітрозативного i оксидативного стресу у виникненні анемії запалення в дітей раннього віку. Матеріали та методи. Визначали вміст нітротирозину та фосфоліпази А2 в сироватці крові 55 дітей раннього віку (середній вік — 1,6 ± 0,3 року) за допомогою імуноферментного аналізу. Основну групу становили 30 дітей із гострими бактеріальними захворюваннями респіраторного тракту, серед яких у 21 пацієнта був діагностований гострий бактеріальний бронхіт i в 9 — позагоспітальна пневмонія. Пацієнти основної групи були розділені на дві підгрупи: перша — 15 дітей із анемією запалення, друга — 15 дітей iз гострими бактеріальними захворюваннями респіраторного тракту без проявів анемії. Групу порівняння становили 10 дітей iз залізодефіцитною анемією без проявів запальних захворювань органів дихання. Контрольна група представлена 15 умовно здоровими дітьми. Результати. Встановлено, що наявність анемії запалення в дітей супроводжується активацією процесів нітрозативного та оксидативного стресу, про що свідчить високий уміст нітротирозину (63,3 ± 4,7 нг/мл), який перевищував показники групи контролю в 5 разів (12,5 ± 1,6 нг/мл) (p < 0,01), та фосфоліпази А2 (6,1 ± 0,7 нг/мл), що був в 2,3 раза (2,28 ± 0,4 нг/мл) (p < 0,05) вищим, нiж у контрольній групі. Визначено позитивну кореляцію між ступенем тяжкості бактеріального запального захворювання та активацією нітрозативного та оксидативного стресу (r = 0,7, p < 0,001). Висновки. Активація кисневмісних та азотовмісних метаболітів на тлі інфекційно-запального захворювання індукує розвиток нітрозативного та оксидативного стресу, що відіграє значну роль у патогенезі анемії запалення в дітей раннього віку iз гострими запальними бактеріальними захворюваннями.
Актуальность. Цель: изучение патогенетической роли нитрозативного и оксидативного стресса в возникновении анемии воспаления у детей раннего возраста. Материалы и методы. Определяли содержание нитротирозина и фосфолипазы А2 в сыворотке крови 55 детей раннего возраста (средний возраст — 1,6 ± 0,3 года) при помощи иммуноферментного анализа. Основную группу составили 30 детей с острыми бактериальными заболеваниями респираторного тракта, среди которых у 21 пациента был диагностирован острый бактериальный бронхит и у 9 — внегоспитальная пневмония. Пациенты основной группы были разделены на две подгруппы: первая — 15 детей с анемией воспаления, вторая — 15 детей с острыми бактериальными заболеваниями респираторного тракта без проявлений анемии. Группу сравнения составили 10 пациентов с железодефицитной анемией без проявлений воспалительных заболеваний органов дыхания. Контрольная группа представлена 15 условно здоровыми детьми. Результаты. Установлено, что наличие анемии воспаления у детей сопровождается активацией процессов нитрозативного и оксидативного стресса, о чем свидетельствует высокое содержание нитротирозина (63,3 ± 4,7 нг/мл), превышавшее показатели группы контроля в 5 раз (12,5 ± 1,6 нг/мл) (p < 0,01), и фосфолипазы А2 (6,1 ± 0,7 нг/мл), которое было в 2,3 раза (2,28 ± 0,4 нг/мл) (p < 0,05) выше, чем в контрольной группе. Установлена положительная корреляция между степенью тяжести бактериального воспалительного заболевания и активацией нитрозативного и оксидативного стресса (r = 0,7, p < 0,001). Выводы. Активация кислородосодержащих и азотсодержащих метаболитов на фоне инфекционно-воспалительного заболевания индуцирует развитие нитрозативного и оксидативного стресса, что играет важную роль в патогенезе анемии воспаления у детей раннего возраста с острыми бактериальными заболеваниями респираторного тракта.
Background. The purpose was to study the pathogenetic role of nitrosative and oxidative stress in the occurrence of anemia of inflammation in young children. Materials and methods. The content of nitrotyrosine and phospholipase A2 in the blood serum of 55 young children (the average age of 1.6 ± 0.3 years) was determined by the method of enzyme-linked immunosorbent assay. The basic group consisted of 30 children with acute bacterial diseases of the respiratory tract: 21 patients were diagnosed with acute bacterial bronchitis, and 9 children — with community-acquired pneumonia. The basic group was divided into two subgroups: the first subgroup consisted of 15 children with anemia of inflammation, the second subgroup — 15 children with acute bacterial diseases of the respiratory tract without anemia manifestation. The comparison group included 10 children with iron deficiency anemia without manifestations of inflammatory diseases of the respiratory system. Fifteen apparently healthy children represented the control group. Results. It was found that anemia of inflammation in children is accompanied by the activation of nitrosative and oxidative stress as evidenced by high nitrotyrosine content (63.3 ± 4.7 ng/ml), which was 5 times greater than in the control group (12.5 ± 1.6 ng/ml) (p < 0.01) and phospholipase A2 level (6.1 ± 0.7 ng/ml), which was 2.3 times higher than in the control group (2.28 ± 0.40 ng/ml) (p < 0.05). The positive correlation was determined between the severity of bacterial inflammatory disease and the activation of nitrosative and oxidative stress (r = 0.7, p < 0.001). Conclusions. The activation of oxygen-containing and nitrogen-containing metabolites against the background of infectious and inflammatory disease induces the development of nitrosative and oxidative stress, which play an important role in the pathogenesis of anemia of inflammation in young children with acute bacterial respiratory diseases.
діти раннього віку; анемія запалення; нітрозативний стрес; оксидативний стрес
дети раннего возраста; анемия воспаления; нитрозативный стресс; оксидативный стресс
young children; anemia of inflammation; nitrosative stress; oxidative stress
Introduction
Over the past decade, much attention has been paid to studying the molecular mechanisms of nitrosative and oxidative stress. These processes are associated with the development and course of a number of mechanisms that are pathogenetic links of inflammatory diseases [1, 2]. Compared to other systems, the respiratory system is the most vulnerable to damage caused by oxidative stress due to anatomical and physiological characteristics. Most diseases of the respiratory tract are accompanied by the intensification of free radical processes at various levels of the biological organization of the body with simultaneous intensification and subsequent inhibition of various parts of the antioxidant defense, which leads to an imbalance in the system of reactive oxygen species and antioxidant defense [1]. The variety of free-radical forms and processes necessitates the selection of specific, highly sensitive, informative markers for their identification and monitoring in bronchopulmonary diseases.
Excessive generation of activated oxygen-containing and nitrogen-containing metabolites can occur both with severe damage to pro-inflammatory cells in response to the effects of pathogen-associated molecular structures of infectious agents or antigens and as a result of exposure to adverse environmental factors [3]. Since the discovery of nitric oxide (NO), an intracellular signal transmitter, its role has been deciphered and systematized [2]. Stable metabolites are formed in the cascade of NO reactions, including nitrotyrosine, a tyrosine nitration product that reflects the activity of protein oxidation [4, 5].
The biochemical manifestations of oxidative stress are an increase in blood levels of superoxide radicals and malondialdehyde, a decrease in the content of ascorbic acid, an increase in the activity of phospholipase A2 and elastase of segmented leukocytes [6]. Gram-negative bacteria contain phospholipase A2 on the outer membrane with a wide range of specificity. It participates in the release of bacteriocin toxin from the cell due to increased membrane permeability with an increase in the level of lysophospholipids and fatty acids in its structure [7]. Cytosolic phospholipase A2 is involved in various cellular processes, but perhaps one of its most noticeable functions is the ability to initiate an inflammatory response: upon hydrolysis of oxidized phospholipids it leads to the formation of inflammatory mediators — lysophosphatidylcholine and oxidized fatty acids [8].
Today, some works demonstrate the relationship between the iron deficiency state and the development of oxidative stress, but so far the pathogenesis of this condition has not been fully studied. It is known that iron is a regulatory factor in the formation of HO and the production of NO as previous metabolites of pathological tyrosine nitration products, including nitrotyrosine. Therefore, iron metabolism disorder induces the progression of oxidative and nitrosative stress. Given the fact that iron sequestration is the basis for the development of anemia of inflammation and is a demonstration of impaired iron metabolism, it leads to an insufficient supply of oxygen to tissues, which, in turn, causes an increase in the concentration of inflammatory mediators, in response to which the generation of activated oxygen-containing and nitrogen-containing metabolites occurs that results in increased nitrosative and oxidative stress [9].
The purpose was to study the pathogenetic role of nitrosative and oxidative stress in the occurrence of anemia of inflammation in young children.
Materials and methods
Fifty-five children aged 1 month to 3 years (the average age was 1.6 ± 0.3 years) were under the supervision. The basic group consisted of 30 children with acute inflammatory bacterial diseases of the respiratory tract: 21 patients (70 %) had acute bronchitis and 9 persons (30 %) — community-acquired pneumonia. Among pathogens, Haemophilus influenzae prevailed in 14 children (46.6 %), Streptococcus pneumoniae — in 9 (30 %), Klebsiella pneumoniae — in 4 (13 %). Other pathogens were identified in isolated cases. Given the hematological picture, the basic group was divided into two subgroups. The first subgroup included 15 children with anemia of inflammation, which was determined 4–5 days after the onset of the disease by a general blood test. The second subgroup consisted of 15 apparently without anemia. The comparison group is represented by 10 children with iron deficiency anemia without inflammatory manifestations. The control group included 15 apparently healthy children. The observation groups were matched by age and sex of the children.
The study of the microbial biomaterials from the mucous membranes of the oropharynx was carried out before antibiotic therapy was prescribed on day 2–3 of the disease using the Vitek 2 Compact bacteriological analyzer (BioMérieux, France) with AES: Global CLSI-based + Phenotypic software.
An enzyme-linked immunosorbent assay (ELISA) determined the content of nitrotyrosine and phospholipase A2 in the blood serum of the examined children. For the study, commercial kits were used: Nitrotyrosine, ELISA (Hycult biotech) and Lipoprotein-associated Phospholipase A2 ELISA.
Assessment of the severity of the condition of patients with inflammatory respiratory diseases was carried out using the Acute Bronchitis Severity Score and Pediatric Respiratory Severity Score.
The obtained results were processed by the method of variation statistics using the statistical packages Excel and Statistica 13.0 (StatSoft Inc., No. JPZ8041382130ARCN10-J). To assess the differences in indicators in the compared groups, Student’s t-test was used. Differences were considered significant at p < 0.05.
All procedures performed in studies involving human participants were under the ethical standards of the institutional and national research committee and with Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study. The full data set by children, their parents, and physician that support the findings of this study are not publicly available due to the restrictions of the ethics approval originally obtained.
Results
The results of the studies are presented in Table 1.
As can be seen from the data given in Table 1, inflammatory bacterial processes in the bronchopulmonary system in young children were accompanied by activation of nitrosative stress, it looks quite logical. The highest activity of the process was noted in the first subgroup of children, where the content of nitrotyrosine increased by 5 times compared to the control group. Against this background, the content of nitrotyrosine in the blood serum of children of the second subgroup increased by 2 times vs control group. The data obtained suggest the pathogenetic role of functional iron deficiency, which is observed in children with anemia of inflammation, in supporting and fully activating nitrosative stress in the presence of an inflammatory process. At the same time, iron deficiency without acute inflammatory process is not a factor in the activation of nitrosative stress, which we observed in children of the comparison group (p < 0.05). We found similar indications after evaluating the serum phospholipase A2, a marker of oxidative stress activity. However, certain differences were present. Firstly, we drew attention to the fact that the activation of oxidative stress was significantly less pronounced. That is, the content of phospholipase A2 in the blood serum of children of the first subgroup exceeded the indicator of the control group by 2.3 times, remaining significantly higher than in children of the second subgroup. Secondly, the fact of the absence of a statistical difference between the indicators obtained from the children of the second subgroup and the data of the comparison group attracted attention. Thus, the data presented may indicate that the activation of nitrosative (primarily) and oxidative stress is an important pathogenetic link in the development of anemia of inflammation.
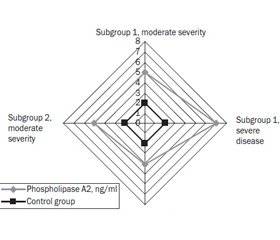
When analyzing the obtained indicators of nitrotyrosine and phospholipase A2 in the blood serum of children of the main study group, taking into account the severity of the disease, the following was established. As can be seen from Fig. 1, there was a direct dependence of the content of nitrotyrosine in the blood serum on the severity of the disease (r = 0.7, p < 0.001). The level of nitrotyrosine in children from the first subgroup with severe disease was 74.25 ± 2.80 ng/ml and almost twice exceeded that of children with moderate severity (38.50 ± 2.43 ng/ml) (p < 0.01), in the second subgroup it was 2.5 times higher (40.1 ± 4.6 ng/ml and 16.1 ± 4.8 ng/ml, respectively) (p < 0.01).
It should be noted that given the severity of the disease, the content of phospholipase A2 in the main observation group is indicative, because in the first subgroup with a severe degree of the disease, it was 7.3 ± 0.4 ng/ml and exceeded that of children with a moderate severity of disease by 1.5 times (4.7 ± 0.7 ng/ml) (p < 0.01), in the second subgroup — by 1.4 times (5.5 ± 0.3 ng/ml and 3.8 ± 0.4 ng/ml, respectively) (p < 0.05) (Fig. 2).
Discussion
The obtained data confirmed the importance of nitrotyrosine and phospholipase A2 in the formation of the inflammatory reaction in young children with inflammatory bacterial diseases of the respiratory system, their influence on nitrosative and oxidative stress, and the role of the latter in the development of anemia of inflammation. Identified violations develop against the background of increased content of the above compounds in the blood serum. This is due to the fact that excessive production of NO during inflammatory processes in the body leads to the formation of its metabolites, including peroxynitrite and nitrogen dioxide. In conditions of inflammation, when a superoxide anion is formed, NO is rapidly depleted in response to a reaction with superoxide, the result of which is the formation of highly reactive peroxynitrite — an extremely powerful oxidant, which is largely responsible for the adverse effects of excessive NO synthesis, causing tissue damage and formation of tyrosine nitration residues [10, 11]. Given the role of this pathogenesis link, our study demonstrates an excess of nitrotyrosine formation during anemia of inflammation, which developed against the background of a major inflammatory disease of the respiratory system. In addition, oxidative stress induced by iron deficiency anemia can be caused by insufficient oxygen supply to the tissues, which leads to an increase in the concentration of inflammatory mediators that activate white blood cells [9, 12]. This process creates favorable conditions for the development of anemia of inflammation. According to a study by T.S. Koskenkorva-Frank et al. (2011), NO production is significantly regulated by iron, and the antioxidant enzyme catalase is a heme-containing enzyme [9]. Thus, dysfunction of normal iron homeostasis induces the development of nitrosative and oxidative stresses, which is confirmed by the results of the study.
At the present stage, there is no doubt as to the role of phospholipase А2 as an initiator of the inflammatory response by interfering with the metabolism of fatty acids. Combined events in which phospholipase A2 is involved lead to the activation of caspase-dependent apoptosis in infected macrophages [13]. The development of the infectious process due to the chain of the aforementioned reactions is associated with the result of opposing the anti-apoptotic properties of infectious agents and activation of the physiological death of the infected cell as a component of the body’s defense mechanism [14]. D.J. Macdonald et al. (2015) in their study show that the catalysis of the elimination of the fatty acid residue from phospholipids leads to their conversion into toxic compounds, the functioning of which leads to the dissolution of red blood cells [15]. Significantly higher levels of phospholipase A2 in the blood serum of children with anemia of inflammation, taking into account the protective function of the aforementioned state in response to the progressive multiplication of bacterial pathogens, can be explained by significant violations of the lipid spectrum of erythrocyte membranes, which are likely to be adaptive in nature and do not affect the ability of red blood cells to deform. The detected changes can possibly be both a consequence of the damaging activity of phospholipase A2 and an adaptation mechanism that ensures the optimization of oxygen transfer from red blood cells to tissues under conditions of anemia [9].
Conclusions
1. Nitrosative and oxidative stress is as a specific pathogenetic link in the development of anemia of inflammation in young children with acute inflammatory bacterial diseases and are manifested with the activation of oxygen-containing and nitrogen-containing metabolites against the background of infectious and inflammatory process in children in the basic group.
2. The degree of activation of nitrosative and oxidative stress is directly reflected (r = 0.7, p < 0.001) on the severity of the disease.
Conflicts of interests. Authors declare the absence of any conflicts of interests and their own financial interest that might be construed to influence the results or interpretation of their manuscript.
1. Ricciardolo F.L.M., Caramori G., Ito K. Nitrosative stress in the bronchial mucosa of severe chronic obstructive pulmonary disease. Journal of Allergy and Clinical Immunology. 2005. 116(5). 1028-1035. http://dx.doi:10.1016/j.jaci.2005.06.034.
2. Sugiura H., Ichinose M. Nitrative stress in inflammatory lung diseases. Nitric Oxide. 2011. 25(2). 138-144. http://dx.doi:10.1016/j.niox.2011.03.079.
3. Bancalari E., Claure N., Sosenko I. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Seminars in Neonatology. 2003. 8(1). 63-71. PMID: 12667831.
4. Louhelainen N., Myllдrniemi M., Rahman I., Kinnula V.L. Airway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: current and future perspectives. International Journal of Chronic Obstructive Pulmonary Disease. 2008. 3(4). 585-603. PMID: 19281076.
5. Woodruff P.G. Novel outcomes and end points: biomarkers in chronic obstructive pulmonary disease clinical trials. Proceedings of the American Thoracic Society. 2011. 8(4). 350-355. http://dx.doi:10.1513/pats.201101-015RM.
6. Tsvetikova L.N., Bugrimov D.Yu., Lobeeva N.V. Metabolicheskie faktory formirovaniya patologicheskikh sostoyanij, svyazannykh s izmeneniem oksidativnogo statusa [Metabolic factors of formation of pathological states connected with the change of oxidative status]. Zhurnal anatomii i gistopatologii. Journal of Anatomy and Histopathology. 2015. 4(2). 12-22 [in Russian]. https://doi.org/10.18499/2225-7357-2015-4-2-14-22.
7. Aresti J., El Aidy S. Microbiota and gut neuropeptides: a dual action of antimicrobial activity and neuroimmune response. Psychopharmacology. 2019. 1. 13. https://doi.org/10.1007/s00213-019-05224-0.
8. Shimizu T., Tomioka H. Novel type of antimicrobial mechanism in host macrophages against mycobacterial infections. Nihon Hansenbyo Gakkai Zasshi. 2009. 78. 283-291. PMID: 19803380.
9. Koskenkorva-Frank T.S., Weiss G., Koppenol W., Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radical Biology and Medicine. 2013. 65. 1174-1194. doi: 10.1016/j.freeradbiomed.2013.09.001.
10. Sugiura H., Ichinose M., Tomaki M., Ogawa H., Koarai A., Kitamuro T., Komaki Y. et al. Quantitative assessment of protein-bound tyrosine nitration in airway secretions from patients with inflammatory airway disease. Free Radical Research. 2004. 38. 49-57. http://dx.doi: 10.1080/10715760310001633817.
11. Connelly L., Madhani M., Hobbs A.J. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice a proinflammatory role for eNOS-derived no in vivo. Journal of Biological Chemistry. 2005. 280. 10040-10046. http://dx.doi:10.1074/jbc.m411991200.
12. Grune T., Sommerburg O., Siems W.G. Oxidative stress in anemia. Clinical Nephrology. 2000. 53(1). 18-22. PMID: 10746801T.
13. Mattson M.P., Duan W., Pedersen W.A., Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001. 6(1–2). 69-81. PMID: 11321043.
14. Oved K., Cohen A., Boico O.A. A Novel Host-Proteome Signature for distinguishing between Acute Bacterial and Viral Infections. PLOS ONE. 2015. 10. 3. http://doi: 10.1371/journal.pone.0120012.
15. Macdonald D.J., Boyle R.M., Glen A.C. The development of an ELISA for group IVA phospholipase A2 in human red blood cells. Prostaglandins Leukot. Essent. Fatty Acids. 2015. 94. 43-48. https://doi:10.1016/j.plefa.2014.11.003.

/10-1.jpg)