Международный эндокринологический журнал 8 (72) 2015
Вернуться к номеру
Особенности показателей тиреоидного гомеостаза у больных с метаболическим синдромом в зависимости от индекса массы тела
Авторы: Абрамова Н.О., Пашковская Н.В. - ВГУЗУ «Буковинский государственный медицинский университет», г. Черновцы
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Introduction. Nonthyroidal illness syndrome develops in patients against the background of chronic comorbidity as a result of impaired peripheral conversion of thyroid hormones and is characterized by low levels of triiodothyronine (T3).
Objective of the study: to find out the features of thyroid homeostasis in patients with metabolic syndrome (MS).
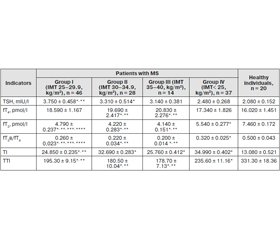
Materials and methods. 64 patients with MS and 20 healthy individuals were involved in the investigation. We determined the level of thyroid stimulating hormone (TSH), free thyroxine (fT4) and free triiodothyronine (fT3). To study the functional status of the pituitary-thyroid axis, we calculated fT3/fT4 ratio and thyroid index (TI). Peripheral activity of thyroid hormones was estimated by total thyroid index (TTI).
Results. In the course of our study, lower fT3 levels and increased levels of TSH and fT4 was revealed in patients with MS compared with the group of healthy subjects (p < 0.05). We found a reduction of fT3/fT4 ratio (p < 0.05) compared to the control group (p< 0.05). TTI was lower in the examined patients compared with the group of healthy individuals (p < 0.05).
As a result of correlation analysis, it was established that body mass index negatively correlated with the level of fT3 (r = –0.341, p < 0.05), fT3/fT4 index (r = –0.458, p < 0.05), TI (r = –0.415, p < 0.05) and TTI (r = –0.335, p < 0.05) and positively — with levels of fT4 (r = 0.405, p < 0.05) and TSH (r = 0.327, p < 0.05).
Conclusion. The obtained data suggests the development of nonthyroidal illness syndrome in patients with metabolic syndrome as a result of impaired peripheral conversion of thyroid hormones, which deepens with body mass index growth, i.e. the class of obesity.
Актуальность. Синдром нетиреоидной патологии развивается у больных на фоне хронической сопутствующей патологии в результате нарушенной периферической конверсии тиреоидных гормонов и характеризуется низким уровнем трийодтиронина (Т3).
Цель исследования: установить особенности тиреоидного гомеостаза у пациентов с метаболическим синдромом (МС).
Материалы и методы. Обследовано 64 пациента с МС и 20 здоровых лиц. Определен уровень тиреотропного гормона (ТТГ), свободного тироксина (сT4) и свободного трийодтиронина (сТ3). Для изучения функционального состояния гипофизарно-тиреоидной оси вычислялось соотношение сT3/сT4 и тиреоидный индекс (ТИ). Периферическую активность тиреоидных гормонов оценивали посредством определения суммарного тиреоидного индекса (СТИ).
Результаты. В ходе нашего исследования более низкие уровни сT3 и повышенное содержание ТТГ и сТ4 выявлено у пациентов с МС по сравнению с группой здоровых лиц (р < 0,05). Обнаружено уменьшение коэффициента сT3/сT4 (р < 0,05) в сопоставлении с группой контроля (р < 0,05). СТИ был ниже у обследованных больных с МС, чем в группе здоровых лиц (р < 0,05).
В результате корреляционного анализа установлено, что индекс массы тела отрицательно коррелирует с уровнем сТ3 (r = –0,341, р < 0,05), соотношением сT3/сT4 (r = –0,458, р < 0,05), TИ (r = –0,415, р < 0,05) и СТИ (r = –0,335, р < 0,05) и положительно — с содержанием сT4 (r = 0,405, р < 0,05) и ТТГ (r = 0,327, р < 0,05).
Вывод. Полученные данные свидетельствуют о развитии синдрома нетиреоидной патологии у пациентов с метаболическим синдромом в результате нарушенной периферической конверсии тиреоидных гормонов, который усугубляется с увеличением индекса массы тела, то есть степени ожирения.
Актуальність. Синдром нетиреоїдної патології (СНТП) розвивається на тлі хронічної супутньої патології в результаті порушеної периферичної конверсії тиреоїдних гормонів і характеризується низьким рівнем трийодтироніну (Т3).
Мета дослідження: встановити особливості метаболізму тиреоїдних гормонів у пацієнтів з метаболічним синдромом (МС).
Матеріал і методи. Обстежені 64 пацієнти з МС і 20 здорових осіб. Визначався рівень тиреотропного гормону (ТТГ), вільного тироксину (вT4) і трийодтироніну (вТ3). Для вивчення функціонального стану гіпофізарно-тиреоїдної осі обчислювалося співвідношення вT3/вT4 і тиреоїдний індекс (ТІ). Периферичну активність тиреоїдних гормонів оцінювали за допомогою визначення сумарного тиреоїдного індексу (СТІ).
Результати. У ході нашого дослідження більш низькі рівні вT3 і підвищений вміст ТТГ і вТ4 виявлено в пацієнтів з МС порівняно з групою здорових осіб (р < 0,05). Встановлено зниження коефіцієнту вT3/вT4 (р < 0,05) порівняно з групою контролю (р < 0,05). СТІ був нижчим у обстежених хворих із МС, ніж у групі здорових осіб (р < 0,05).
У результаті кореляційного аналізу було встановлено, що індекс маси тіла негативно корелює з рівнем вТ3 (r = –0341, р < 0,05), співвідношенням вT3/вT4 (г = –0458, р < 0,05), ТІ (r = –0415, р < 0,05) і СТІ (r = –0335, р < 0,05) і позитивно — з вмістом вT4 (R = 0,405, р < 0,05) і ТТГ (r = 0327, р < 0,05).
Висновок. Отримані дані свідчать про розвиток синдрому нетиреоїдної патології в результаті порушеної периферичної конверсії тиреоїдних гормонів у пацієнтів з метаболічним синдромом, що поглиблюється із зростанням індексу маси тіла, тобто ступеня ожиріння.
obesity, hypertension, metabolic syndrome, thyroid homeostasis, nonthyroidal illness syndrome.
ожирение, артериальная гипертензия, метаболический синдром, тиреоидный гомеостаз, синдром нетиреоидной патологии.
ожиріння, артеріальна гіпертензія, метаболічний синдром, тиреоїдний гомеостаз, синдром нетиреоїдної патології.
The article was published on p. 27-30
Nonthyroidal illness syndrome (NTI), also known as syndrome of low triiodothyronine (T3), occurs against a background of chronic concomitant diseases and is characterized by reduction of triiodothyronine due to inhibition of deiodinases — enzymes which catalyze peripheral conversion of thyroxine (T4) to its active metabolite (T3). These changes are typical for 75 % of hospitalized patients [10, 11]. About 80 % of thyroid hormones are produced in peripheral tissues through the activity of deiodinases [5, 13].
Material and methods
Results
Discussion
Conclusions
1. Зарецкий М.М. Метаболический синдром в клинической практике / М.М. Зарецкий, Н.М. Черникова, Т.В. Лобачевская // Новости медицины и фармации. — 2010. — № 17(340). — С. 9-15.
2. Кандор В.И. Синтез, секреция и метаболизм тиреоидных гормонов / В.И. Кандор под ред. Н.Т. Старковой // Руководство по клинической эндокринологии. — СПб.: Питер-пресс, 1996. — С. 115-124.
3. Старкова Н.Т. Клиническая эндокринология / Н.Т. Старкова. — М.: Медицина. — 1991. — С. 151-153.
4. Araujio R.L. Tissue-specific deiodinase regulation during food restriction and low replacement dose of leptin in rats / R.L. Araujio, B.M. Andrade // Am. J. Physiol .Endocinol. Metab. — 2009. — Vol. 296. — P. 1157-1163.
5. Bello G. The role of thyroid disfunction in critically ill: a review of the literature // G. Bello, I. Ceaichisiuc // Minerva anestesiologica. — 2010. — Vol. 76, № 11. — P. 919-928.
6. Circulating leptin and thyroid dysfunction / T. Zimmermann-Belsing, G Brabant, J.J. Holst [et al.] // Eur. J. Endocrinol. — 2003. — Vol. 149, № 4. — P. 257-271.
7. Dentice M. Deiodinases: the balance of thyroid hormone: local impact of thyroid hormone inactivation / M. Dentice, D. Salvatore // J. Endocrinol. — 2011. — Vol. 209, № 3. — P. 273-282.
8. Does extreme obesity affect tyroid hormone metabolism? / M.A. Michalaki, M.I. Gkotsina, I. Mamali [et al.] // Clinical thyroidology. — 2011. — Vol. 23, № 6. — P. 9-10.
9. Kelly G. Peripheral metabolism of thyroid hormones: A review / G. Kelly // Alternative medicine review. — 2000. — Vol. 5, № 4. — P. 306-333.
10. Myers Adler S. The nonthyroidal illness syndrome / S. Myers Adler, L. Wartofsky // Endocrinol Metab Clin. N. Am. — 2007. — Vol. 36 — P. 657-672.
11. Pappa T.A. The nonthyroidal illness syndrome in the non-critically ill patient / T.A. Pappa, A.G. Vagenacis // European journal of clinical investigation. — 2011. — Vol. 41, № 2. — P. 212-220.
12. Shanta. G. Association between primary hypothyroidism and metabolic syndrome and the role of C reactive protein: a cross-sectional study from South India / G. Shanta, A. Kumar // Thyroid Research. — 2009. — Vol. 2, № 2. — P. 175-185.
13. Williams Graham R. Local control of thyroid hormone action: role of type 2 deiodinase Deiodinases: the balance of thyroid hormone / Graham R. Williams, J.H. Duncan Bassett // Journal of Endocrino–logy. — 2011. — Vol. 209. — P. 261-272. — doi: 10.1530.
1. Zaretskyy M.M. Chernykova N.M., Lobachevskaya T.V. [Metabolic syndrome in clinical practice]. News of Medicine and Pharmacy. 2010;17(340):9 – 15. Russian
2. Kandor V.Y., author, Starkova N.T., editor. Syntez, sekretsyya y metabolyzm tyreoydnыkh hormonov: Rukovodstvo po klynycheskoy эndokrynolohyy [Synthesis, secretion and metabolism of thyroid hormones: A guide to clinical endocrinology]. N.t.-spb.: Pyter press;1996.115 – 124. Russian
3. Starkova N.T. Klynycheskaya эndokrynolohyya [Clinical endocrinology] M.: Medytsyna;1991.151–153.
4. Araujo RL, Andrade BM, da Silva ML, et al. Tissue-specific deiodinase regulation during food restriction and low replacement dose of leptin in rats. Am J Physiol Endocinol Metab. 2009;296:E1157-E1163.
5. Bello G., Ceaichisiuc I, Silva S, Antonelli M. The role of thyroid disfunction in critically ill: a review of the literature. Minerva anestesiol. 2010;76:919 – 928a.
6. Zimmermann-Belsing T, Brabant G, Holst J.J., Feldt-Rasmussen U. Circulating leptin and thyroid dysfunction. Eur J Endocrinol. 2003; 149(4):257 – 271.
7. Dentice M, Salvatore D. Deiodinases: the balance of thyroid hormone: local impact of thyroid hormone inactivation. J Endocrinol. 2011; Jun;209(3):273-82.
8. Michalaki MA, Gkotsina MI, Mamali I, Markantes GK, Faltaka A, Kalfarentzos F, Vagenakis AG, Markou KB. Does extreme obesity affect tyroid hormone metabolism? Clinical thyroidology. 2011;23 (6): 9 – 10.
9. Kelly G, Peripheral Metabolism of Thyroid Hormones: A Review. Alt Med Rev. 2000;5(4):306-333.
10. Myers Adler S., Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin N Am. 2007;36:657 – 672.
11. Pappa T. A., Vagenacis A.G. The nonthyroidal illness syndrome in the non-critically ill patient. European journal of clinical investigation. 2011;41(2): 212 – 220.
12. Shanta. G., Kumar A. Association between primary hypothyroidism and metabolic syndrome and the role of C reactive protein: a cross-sectional study from South India. Thyroid Research. 2009;2(2):175 – 185.
13. Williams Graham R , Duncan Bassett J. H. Local control of thyroid hormone action: role of type 2 deiodinase Deiodinases: the balance of thyroid hormone. Journal of Endocrinology. 2011;209:261 – 272.

/29.jpg)